Anti-androgens for the treatment of metastatic castration-sensitive prostate cancer
A prostate cancer and anti-androgen technology, applied in the direction of anti-tumor drugs, organic active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
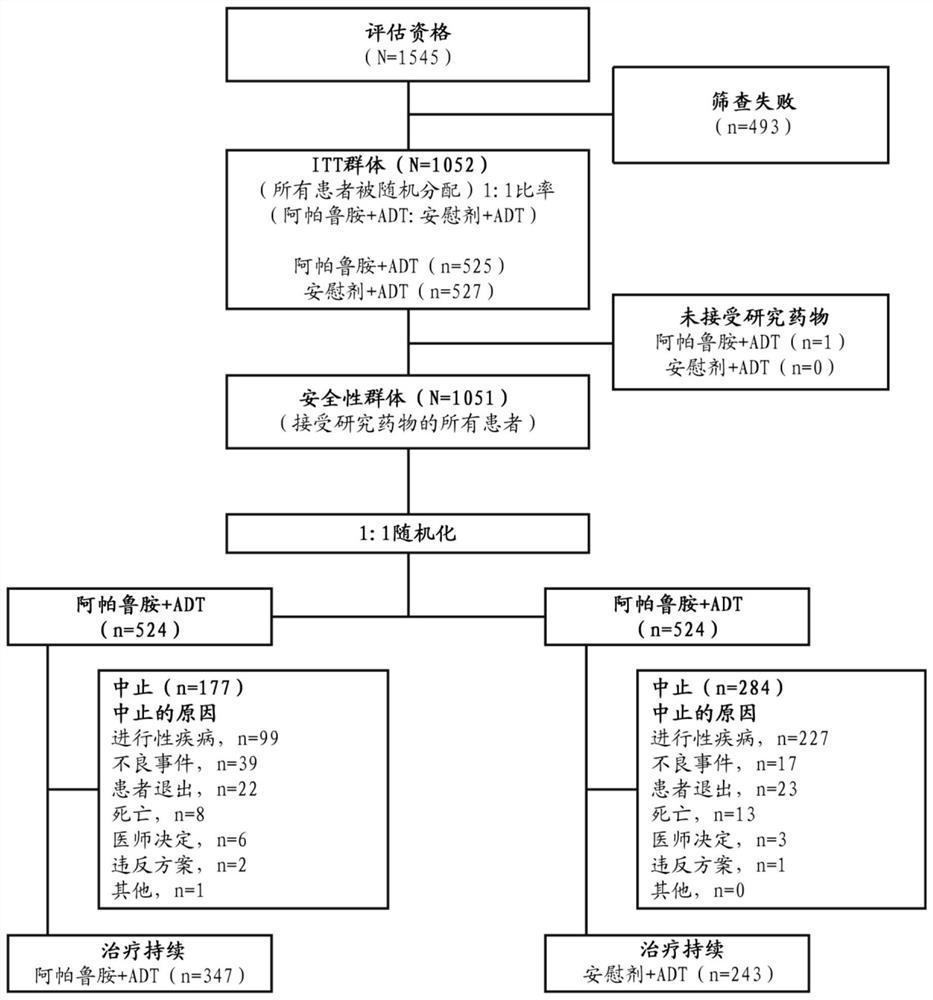
[0221] Example 1: Apalutamide plus androgens in subjects with metastatic hormone-sensitive prostate cancer (mCSPC) Phase 3 randomized, placebo-controlled, double-blind study of resection therapy (ADT) versus ADT
[0222] Target
[0223] main target
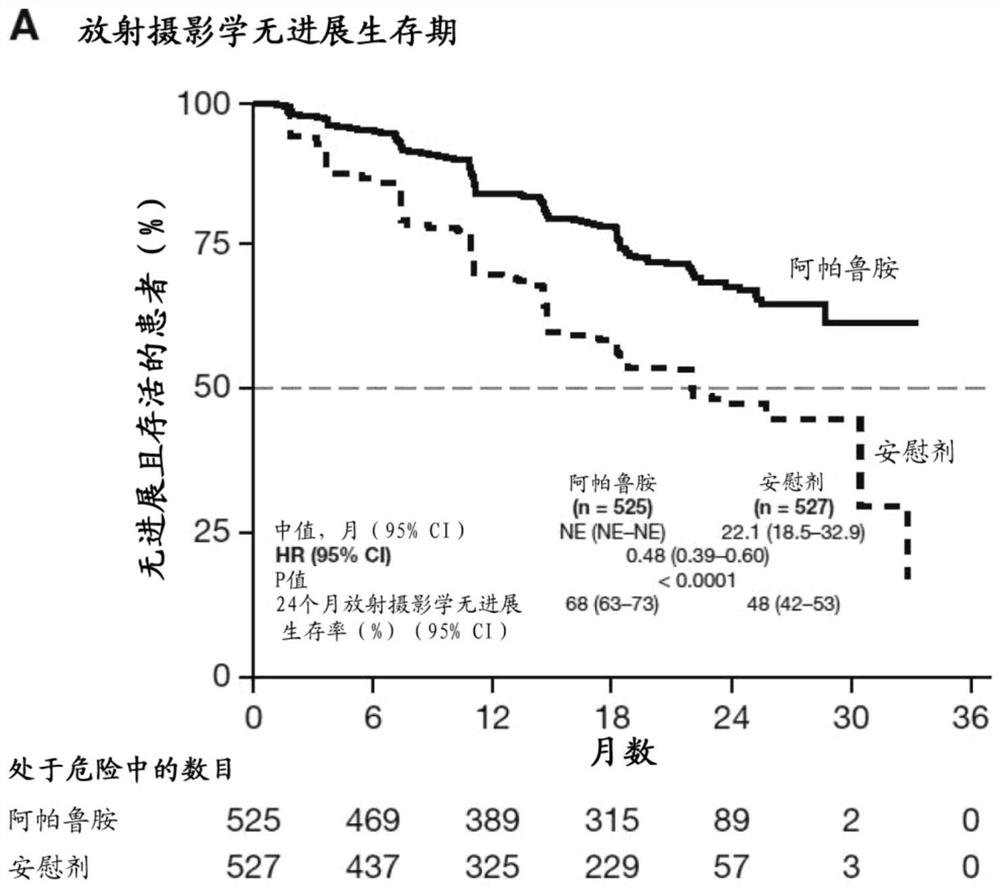
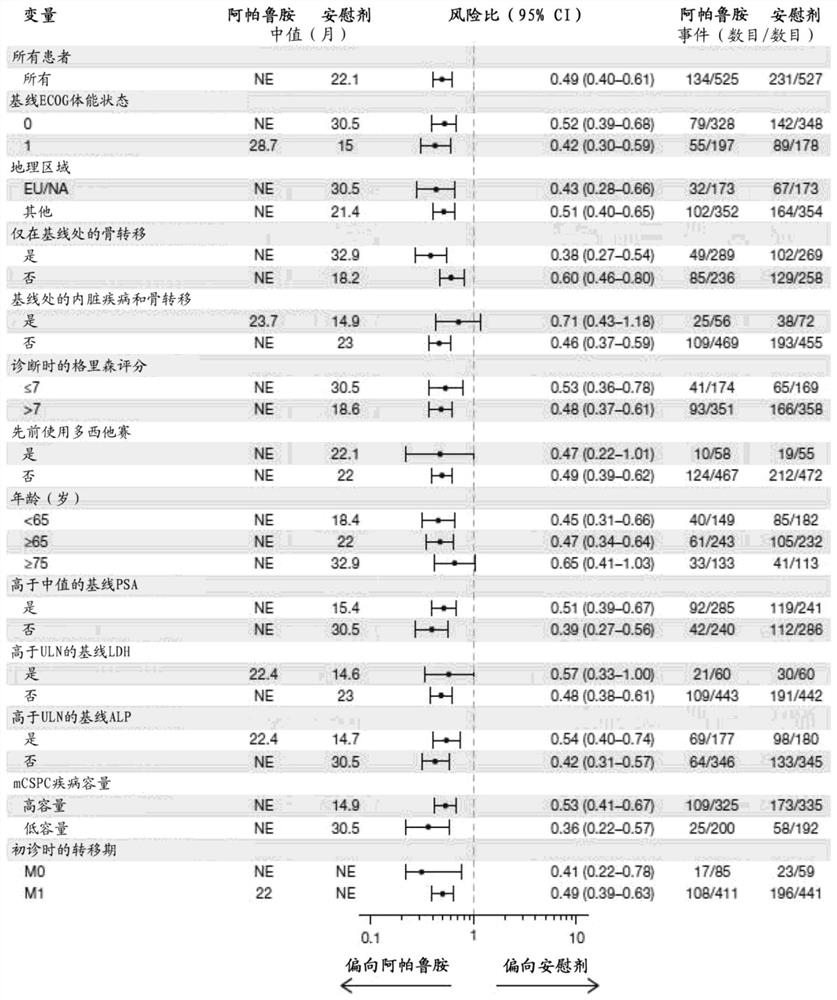
[0224] To determine whether the addition of apalutamide to androgen deprivation therapy (ADT) provides superior efficacy in prolonging radiographic progression-free survival (rPFS) or overall survival (OS) in subjects with mCSPC.
[0225] secondary goal
[0226] Evaluate clinically relevant improvements caused by the addition of apalutamide to ADT, including delays in pain progression and opioid use for prostate cancer, skeletal-related events, and the need for cytotoxic chemotherapy;
[0227] Characterize the safety of adding apalutamide to ADT for subjects with mCSPC;
[0228] · Characterization of the population pharmacokinetics (PK) and pharmacodynamics (PD) of apalutamide;
[0229] assess the concentration of le...
Embodiment 2
[0514] Example 2: Final FDA Approved Drug Product Labeling
[0515] FDA approved ERLEADA on September 17, 2019 TM The following drug product labels for (apalutamide), ERLEADA TM is a reference listed drug for apalutamide.
[0516]
[0517]
[0518] Full Prescribing Information
[0519] 1. Indications and usage
[0520] ERLEADA is indicated for the treatment of patients with:
[0521] Metastatic castration-sensitive prostate cancer (mCSPC)
[0522] Non-metastatic castration-resistant prostate cancer (nmCRPC)
[0523] 2 Dosage and Administration
[0524] 2.1 Recommended dosage
[0525] The recommended dose of ERLEADA is 240 mg (four 60 mg tablets) administered orally once daily. Swallow the tablet whole. ERLEADA can be taken with or without food.
[0526] Patients should also be receiving concomitant gonadotropin-releasing hormone (GnRH) analogs or should have undergone bilateral orchiectomy.
[0527] 2.2 Dosage adjustment
[0528] If the patient experience...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com