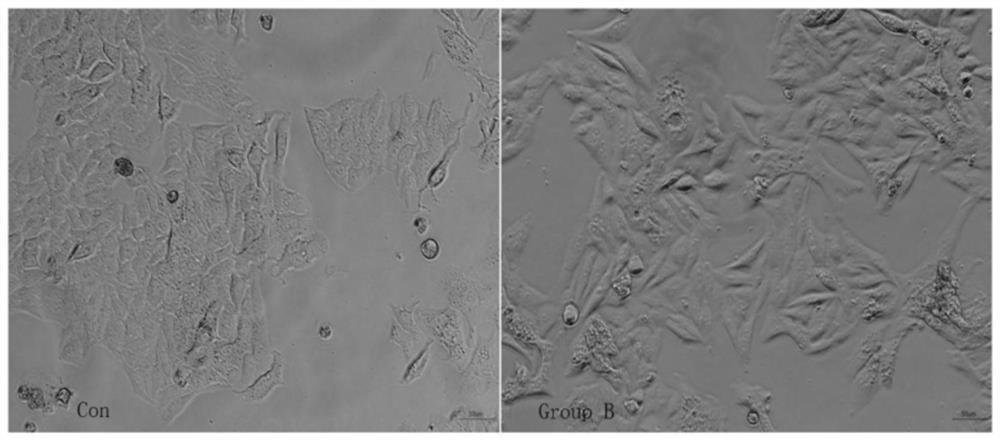
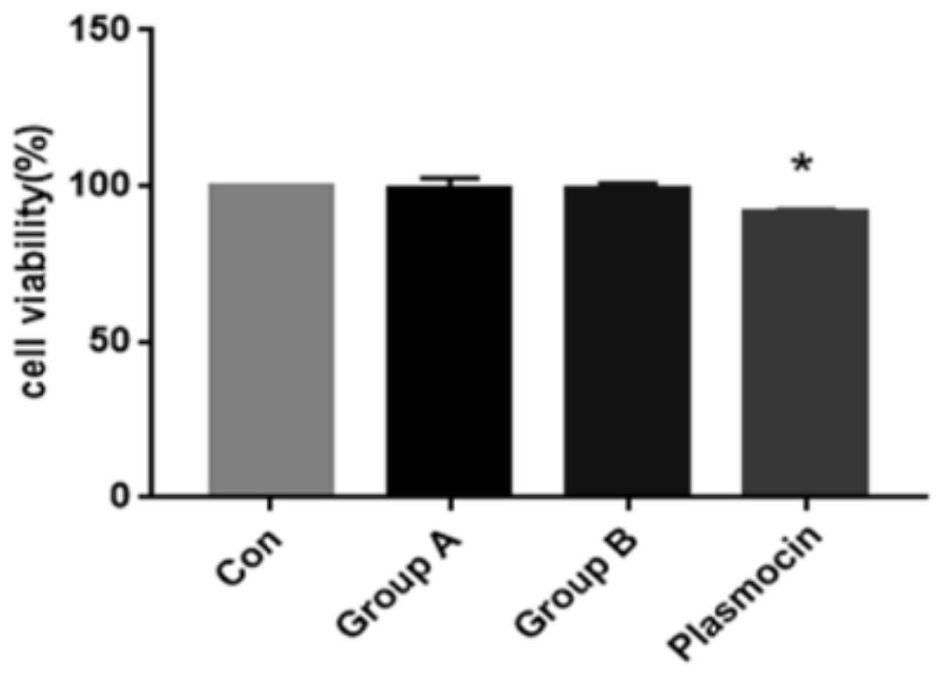
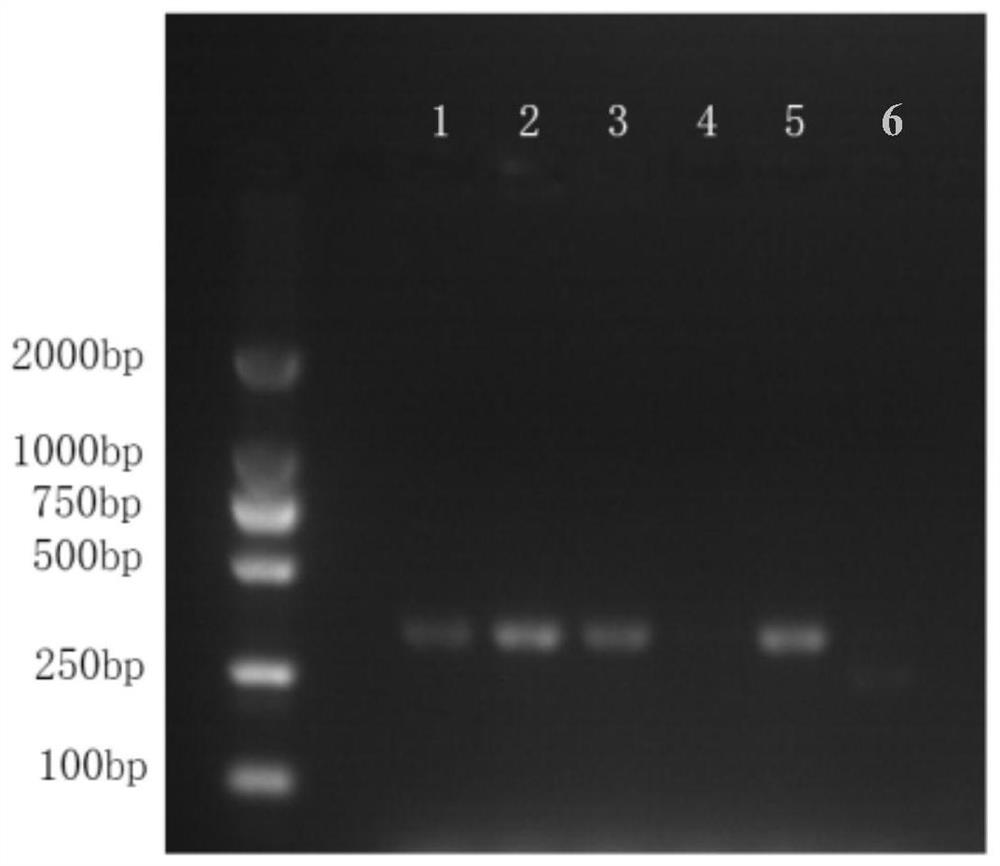
Mycoplasma removal composition and method for treating in-vitro cultured cells
A technology for removing compositions and mycoplasma, applied in biochemical equipment and methods, tissue culture, animal cells, etc., can solve problems such as time-consuming, pollution recurrence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In the mycoplasma removal composition 1 of the present application, the final concentrations of erythromycin, chloramphenicol and tylosin are respectively 20 μM, and the final concentration of ciprofloxacin is 4 μg / ml. When used, the DMEM cell culture medium was used as the base medium, containing 10% fetal bovine serum.
Embodiment 2
[0041] In the mycoplasma removal composition 2 of the present application, the final concentrations of erythromycin, chloramphenicol and tylosin are respectively 20 μM, and the final concentrations of ciprofloxacin are 4 μg / ml. When used, MEM cell culture medium is used as the base medium, containing 10% fetal bovine serum.
Embodiment 3
[0043] In the mycoplasma removal composition 3 of the present application, the final concentrations of erythromycin, chloramphenicol and tylosin are respectively 40 μM, and the final concentrations of ciprofloxacin are 4 μg / ml. When used, the DMEM cell culture medium was used as the base medium, containing 10% fetal bovine serum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com