Preparation method, production equipment and use method of 2, 4, 6-trichloroaniline
A technology of trichloroaniline and production equipment, which is applied in the field of preparation of 2,4,6-trichloroaniline, can solve the problems of high enterprise cost and low effective conversion rate, and achieve enterprise cost saving, low consumption, and improved product synthesis rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of 2,4,6-trichloroaniline disclosed by the invention comprises the following steps:
[0045] Put chlorobenzene and aniline into the reaction kettle for synthesis, then pass into hydrogen chloride gas, and finally pass into chlorine gas;
[0046] After passing the chlorine gas, keep warm until the reaction is complete, filter to obtain the filter cake and filtrate, press filter the filter cake and dry to obtain 2,4,6-trichloroaniline; the filtrate is chlorobenzene;
[0047] Add the hydrogen chloride gas produced in the reaction kettle for synthesis and the chlorobenzene obtained by filtering into the reaction kettle for acidification, use the hydrogen chloride gas to acidify the chlorobenzene, and then add the acidified chlorobenzene into the reaction kettle for synthesis React provides a reactive environment.
[0048] The invention reuses the tail gas (hydrogen chloride gas) produced by the synthesis reaction, which can provide a better acidic en...
Embodiment 1
[0051] In this embodiment, the original single reactor is designed into a two-stage closed environment, and the tail gas generated by the first-stage reactor is recycled to improve the reaction efficiency and the product synthesis rate.
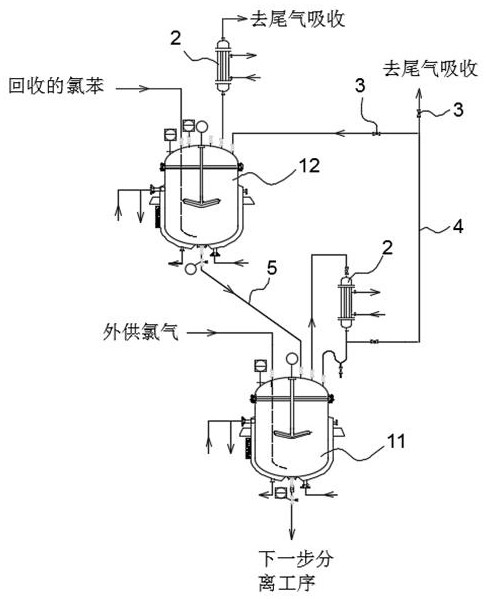
[0052] Specific as figure 1 As shown, the production equipment of 2,4,6-trichloroaniline disclosed in this embodiment includes a primary enamel reactor 11 and a secondary enamel reactor 12, the primary enamel reactor 11 is used for synthesis reaction, and the secondary enamel reactor 11 is used for synthesis reaction. Reactor 12 is used for acidification.
[0053]The secondary enamel reaction kettle 12 is located above the primary enamel reaction kettle 11 . The product outlet at the bottom of the secondary enamel reactor 12 is connected to the primary enamel reactor 11 through a valve and a liquid pipeline 5 .
[0054] The gas outlet of the primary enamel reaction kettle 11 is connected with the secondary enamel reaction kettle 12 through ...
Embodiment 2
[0065] In this embodiment, the original single reactor is designed into a three-level closed environment, and the tail gas generated by the first-level reactor is recycled to improve the reaction efficiency and the product synthesis rate.
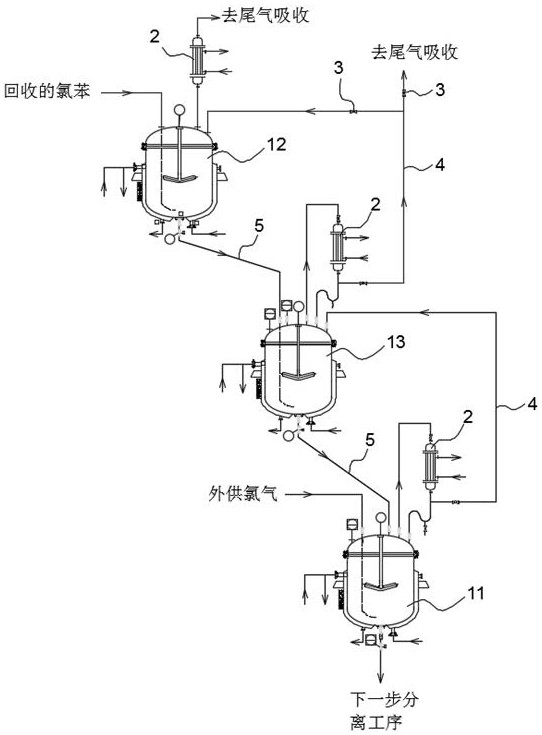
[0066] like figure 2 As shown, the production equipment of 2,4,6-trichloroaniline disclosed in this embodiment includes a primary enamel reactor 11, a secondary enamel reactor 12 and a tertiary enamel reactor 13, and the primary enamel reactor 11 is used as For the synthesis reaction, the secondary enamel reactor 12 is used for primary acidification, and the tertiary enamel reactor 13 is used for secondary acidification.
[0067] The third-level enamel reaction kettle 13 is located above the first-level enamel reaction kettle 11 , and the second-level enamel reaction kettle 12 is located above the third-level enamel reaction kettle 13 .
[0068] The product outlet at the bottom of the secondary enamel reaction kettle 12 is connected to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com