Acylated derivative of mogrolic acid and preparation method thereof
A kind of derivative, Luohan technology, applied in the field of acylation of Luohanguo acid and its preparation field, can solve the problems of short half-life, limited application, poor water solubility of Luohanguo acid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
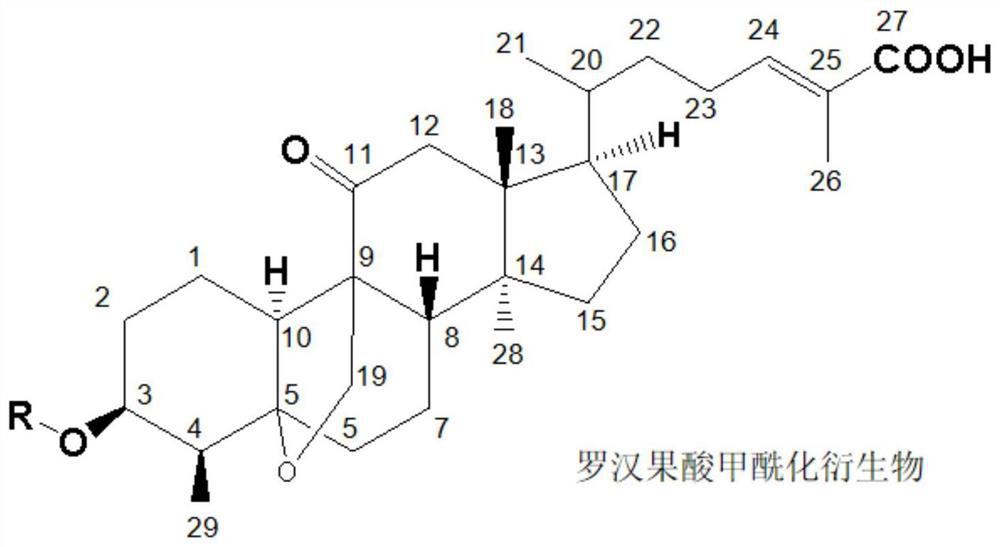
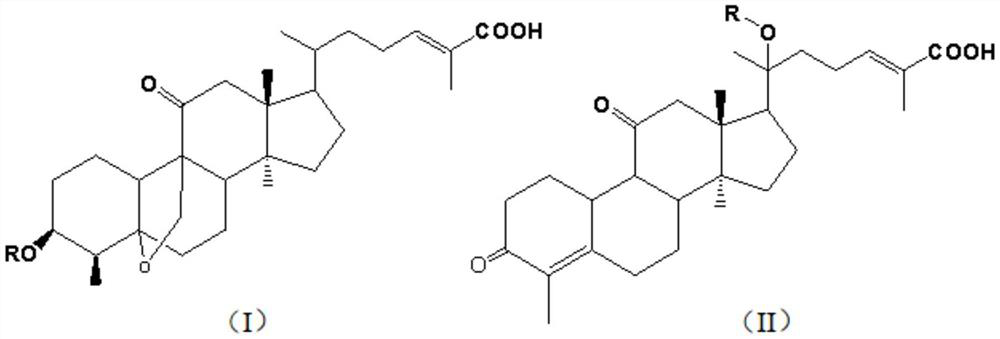
[0034] (1) Acylation reaction: Take 4.72g (0.01mol) of mogrosinic acid A, 2.47g (0.012mol) of DCC, and 1.5g of triethylamine dissolved in 35mL of ethyl acetate, stir for 30min under ice bath, then slowly add 15mL of the solution dropwise. There are 1.33g (0.013mol) of acetic anhydride and 0.12g of DMAP in ethyl acetate solution, the dropwise addition is completed within 1 hour, and the reaction is stirred at room temperature for 8 hours, and the reaction is complete by HPLC detection.
[0035] (2) Post-treatment: After the reaction is complete, the reaction solution is washed with water to neutrality, added with 2.5 g of anhydrous sodium sulfate for dehydration, concentrated under reduced pressure, and dried in vacuo to obtain a white solid. v / v) was the developing solvent, and finally obtained 4.49g of the product as white crystal 3-O-acetylmogrosvenous acid A, with a purity of 98.31% and a yield of 85.78% by HPLC.
[0036]
Embodiment 2
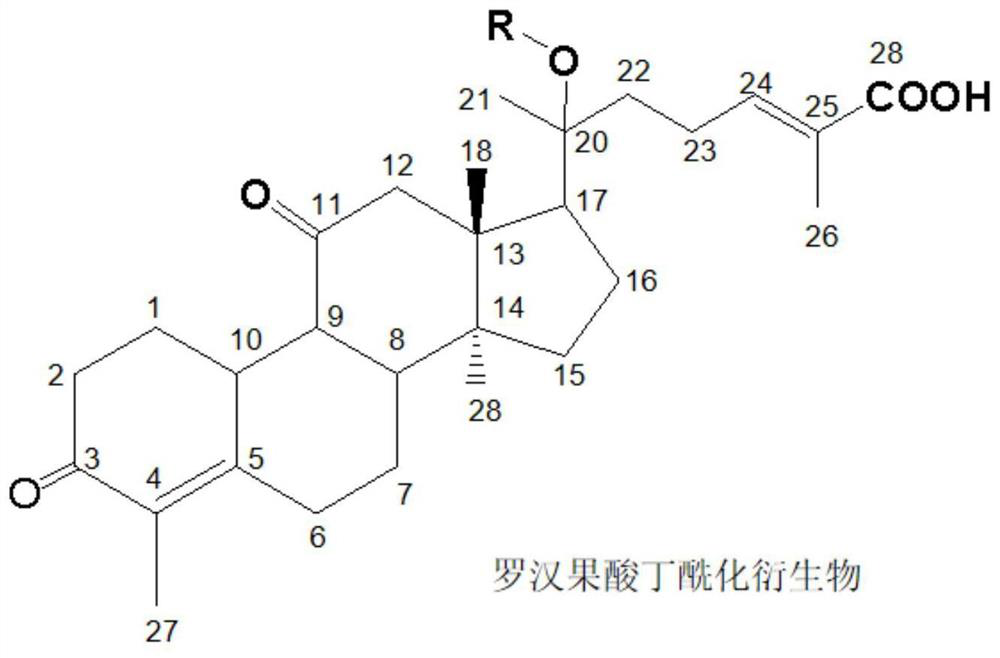
[0038] (1) Acylation reaction: Take 4.56g (0.01mol) of mogrosinic acid butyl, 2.47g (0.012mol) of DCC, 4.2g of pyridine dissolved in 50mL of ethyl acetate, stir for 30min under ice bath, then slowly add dropwise 15mL of 1.33 g (0.013mol) of acetic anhydride and 0.12g of DMAP in ethyl acetate were added dropwise within 1 hour, stirred and reacted at room temperature for 8 hours, and the reaction was complete by HPLC detection.
[0039] (2) Post-treatment: after the reaction is complete, the reaction solution is washed with water to neutrality, added with 3g of anhydrous magnesium sulfate for dehydration, concentrated under reduced pressure, and dried in vacuo to obtain a white solid. / v) was the developing solvent, and finally obtained 4.49g of the product as white crystal 20-O-acetyl mogrosinic acid A, with a purity of 97.54% and a yield of 85.78% by HPLC.
[0040]
Embodiment 3
[0042]Other conditions and operations were the same as in Example 1, except that 1.33 g (0.013 mol) of acetic anhydride was replaced by 1.69 g (0.013 mo) of propionic anhydride. Finally, 4.60 g of white crystal 3-O-propionyl mogrosinic acid A was obtained, with a purity of 98.17% and a yield of 85.45% by HPLC.
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com