Preparation method of carboxymethyl inulin
A technology of carboxymethyl inulin and inulin, which is applied in the field of preparation of carboxymethyl inulin, can solve the problems that the synthesis process cannot expand production, and achieve the effects of good appearance, reduced reaction time, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a kind of preparation method of carboxymethyl inulin, comprising the following steps:
[0025] (1) mix inulin, sodium hydroxide, pyridinium salt and solvent, carry out alkalization reaction, obtain alkalized inulin;
[0026] (2) Mix the alkalized inulin, sodium hydroxide, organic chloride and solvent to carry out etherification reaction to obtain the carboxymethyl inulin.
[0027] In the present invention, the pyridinium salt in the step (1) is preferably N-butylpyridine tetrafluoroborate.
[0028] In the present invention, the solvent in the step (1) is preferably ethanol or isopropanol.
[0029] In the present invention, the volume fraction of the ethanol is preferably 93-97%, more preferably 94-96%.
[0030] In the present invention, the mass ratio of inulin to pyridinium salt in the step (1) is preferably 4-5: 0.05-0.1, more preferably 4.2-4.8: 0.06-0.09, more preferably 4.4-4.6: 0.07- 0.08.
[0031] In the present invention, the dosage ra...
Embodiment 1
[0051] Mix 4.5g of inulin, 1g of sodium hydroxide and 20mL of isopropanol, add 0.1g of N-butylpyridine tetrafluoroborate dropwise at a rate of 55 drops / min, and control the stirring speed to 25rpm after the addition , alkalized at room temperature for 15 minutes to obtain alkalized inulin;
[0052] Keep stirring after obtaining alkalized inulin, continue to add 0.76g of sodium hydroxide; then weigh 2.3g of chloroacetic acid and dissolve it in 10mL of isopropanol, and mix the mixture of chloroacetic acid and isopropanol at a rate of 60 drops / min. Add the mixture dropwise into the reaction system. After etherification reaction at room temperature for 2 hours, suction filter at 0.01 MPa, wash the suction filtered product thoroughly with 95% ethanol, and dry at 40°C for 6 hours after washing to obtain carboxymethyl base inulin.
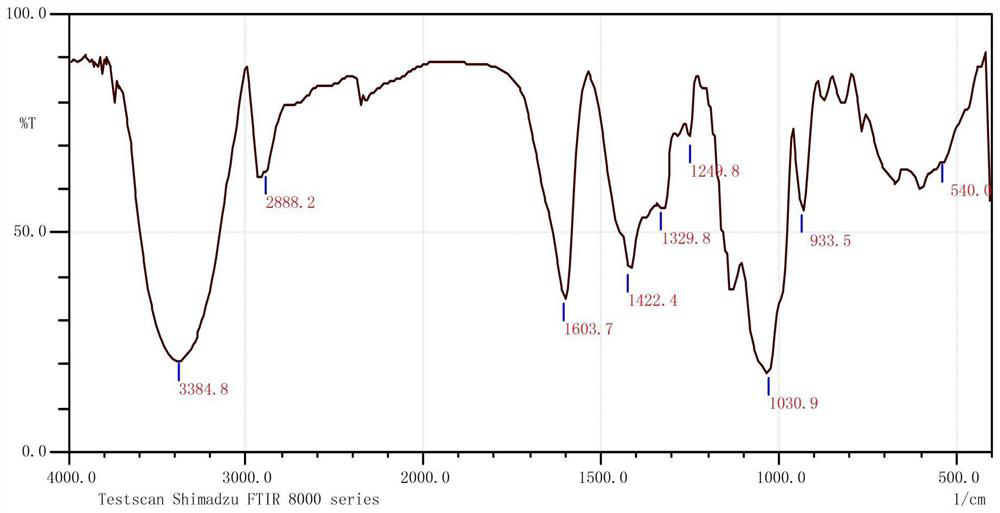
[0053] The degree of substitution of the carboxymethyl inulin prepared in this example is 0.71.
[0054] The infrared spectrum of the carboxymethyl inu...
Embodiment 2
[0056] Take 45g of inulin, 12g of sodium hydroxide and 250mL of 95% ethanol and mix them, and add 0.8g of N-butylpyridine tetrafluoroborate dropwise at a rate of 60 drops / min. Alkalizing reaction at room temperature for 20 minutes to obtain alkalized inulin;
[0057] Keep stirring after obtaining the alkalized inulin, continue to add 8g of sodium hydroxide; then weigh 30g of chloroacetic acid and dissolve it in 120mL of isopropanol, and drop the mixture of chloroacetic acid and isopropanol at a rate of 60 drops / minute Add it into the reaction system, after etherification reaction at room temperature for 2.3h, then suction-filter at 0.01MPa, fully wash the suction-filtered product with 95% ethanol, and dry at 38°C for 6.3h after washing to obtain carboxymethyl base inulin.
[0058] The degree of substitution of the carboxymethyl inulin prepared in this example is 0.71.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com