A kind of method for synthesizing higher alcohol by catalytic conversion of ethanol
A technology for catalytic conversion and higher alcohols, applied in chemical instruments and methods, preparation of organic compounds, carbon-based compounds, etc., can solve the problems of complex catalyst preparation, high price, unfavorable preparation and application, etc., and achieve excellent stability, Effects of low cost, increased selectivity and ethanol conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
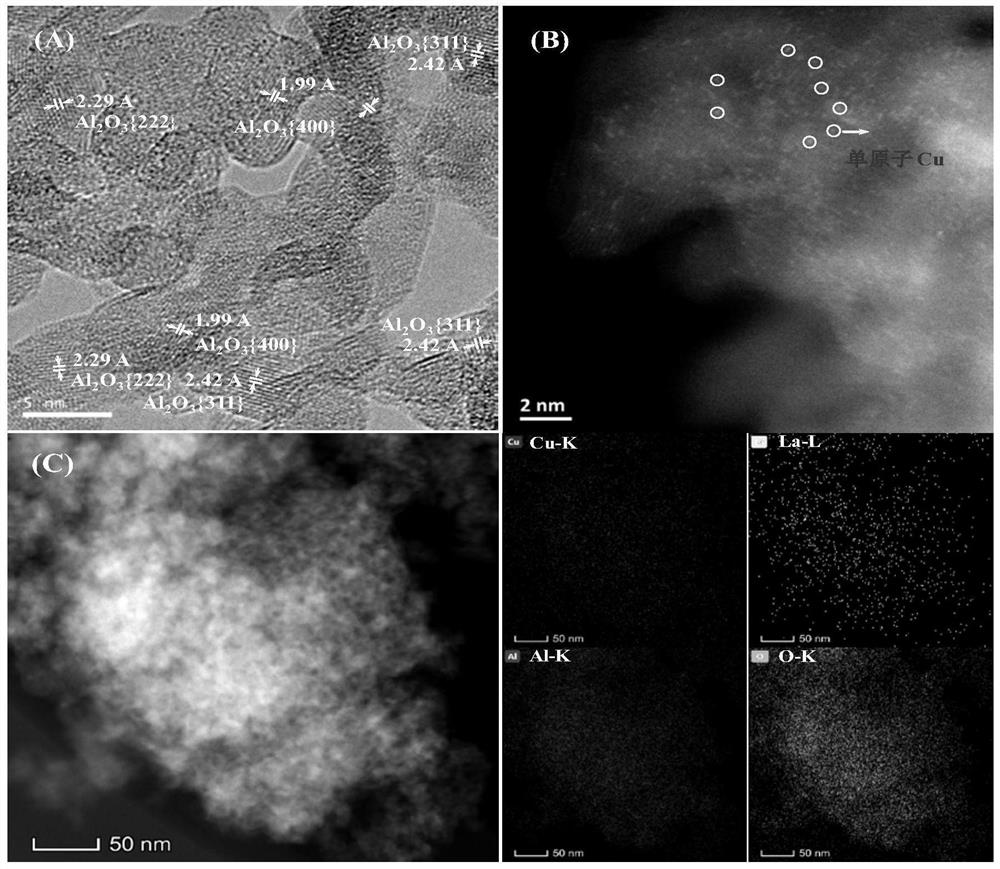
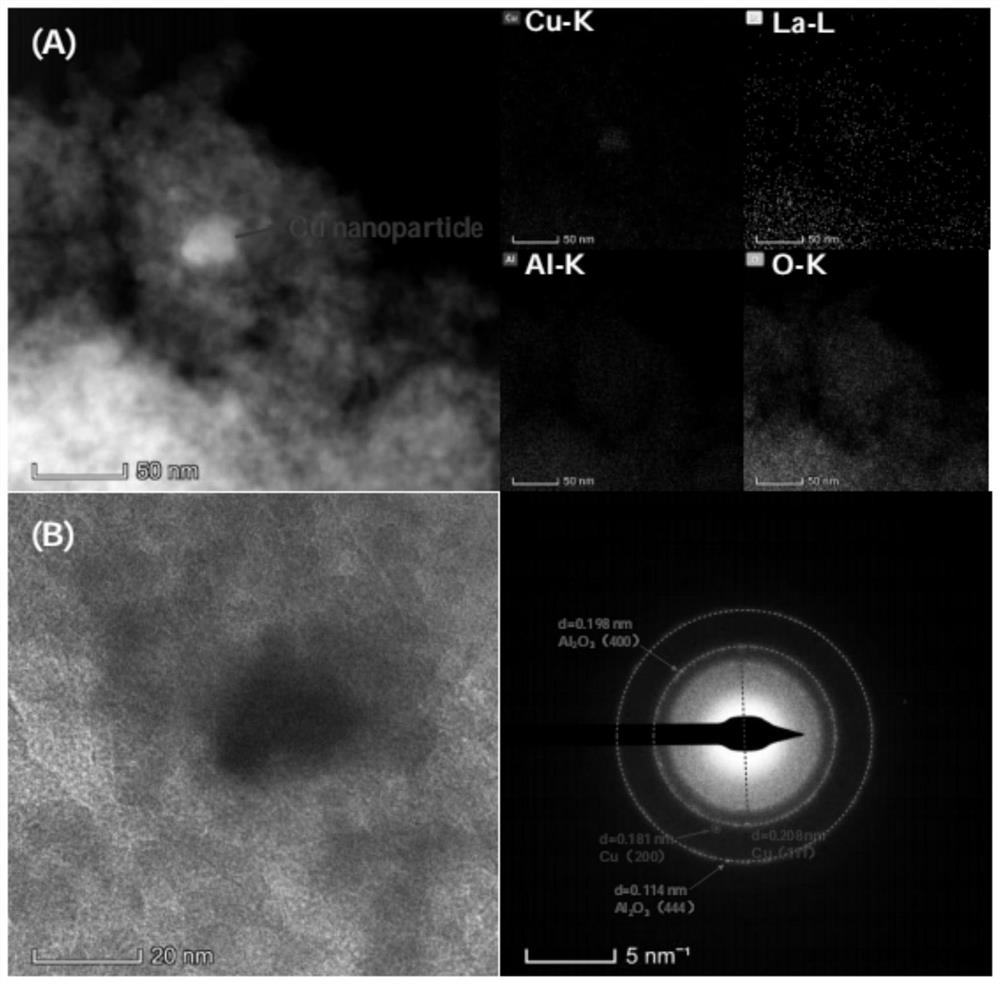
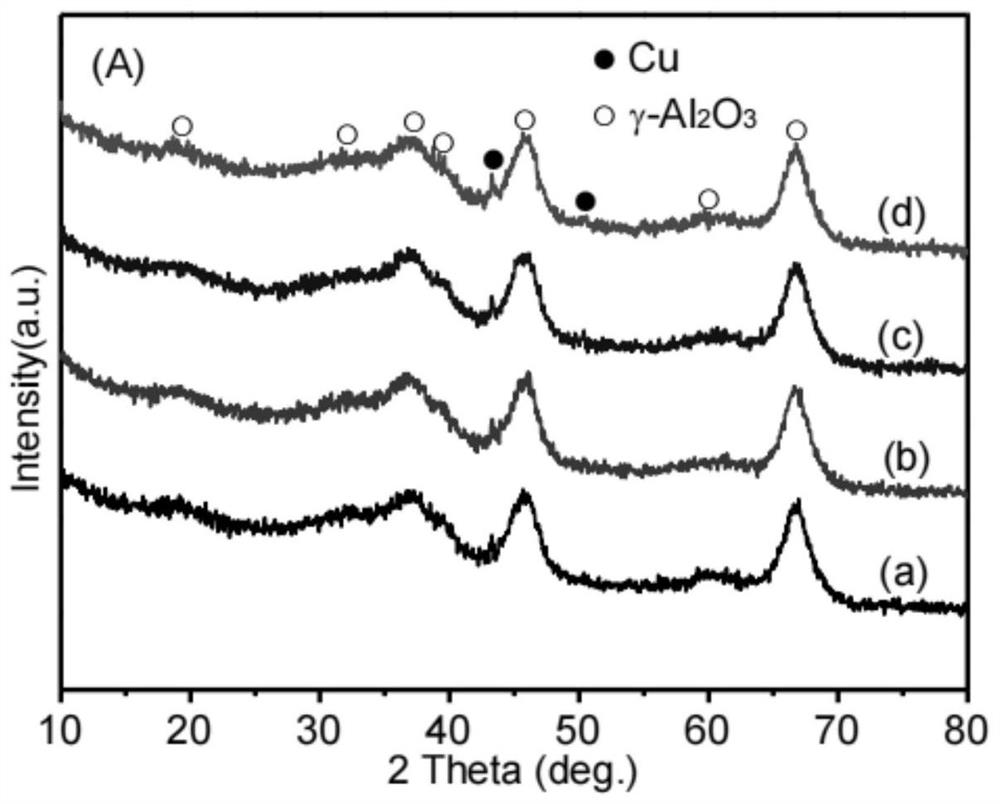
[0069] 0.7603g copper nitrate (Cu(NO 3 ) 2 ·3H 2 O) and 0.4555g cerium nitrate (Ce(NO) 3 ) 3 ·6H 2 O) was added to 10ml of absolute ethanol, after it was dissolved and mixed uniformly, 2g of alumina carrier (specific surface was 291m 2 / g, the average pore size is 10.1 nm, and the pore volume is 0.74 mL / g). The above mixture was dried on a rotary evaporator at 50 °C and 0.09 MPa for 3 h, and then dried at 80 °C and 0.09 MPa for 2 h. The dried solid matter was calcined in a muffle furnace at 450 °C for 3 h in an air atmosphere, and then calcined in a tube furnace or fixed bed reactor with 10% H 2 / N 2 Mixed gas at 500 ℃, gas air velocity 1800h -1 The catalyst I-a is obtained by reducing under the condition for 6h. The weight content of its metal Cu is 8.4wt%, CeO 2 The loading amount is 7.6wt%, and the rest is alumina carrier. The surface element composition and valence state of the catalyst were analyzed by X-ray fluorescence spectroscopy (XPS) characterization tech...
Embodiment 2
[0072] The preparation method of catalyst I-b is the same as in Example 1, but copper nitrate (Cu(NO) 3 ) 2 ·3H 2 O) and cerium nitrate (Ce(NO) 3 ) 3 ·6H 2 The masses of O) were 0.6083 g and 0.4555 g, respectively. The weight content of its metal Cu is 6.8 wt%, CeO 2 The weight content is 7.7wt%, and the rest is alumina carrier. The surface element composition and valence state of the catalyst were analyzed by XPS characterization technology, and it was proved that in the reduced catalyst, cerium was replaced by CeO. 2 form, while the copper active component exists in the form of +1-valent Cu and zero-valent Cu, and Cu 0 / Cu + The ratio (molar ratio) was 1:6.9.
[0073] Continuous catalytic conversion of ethanol to synthesize higher alcohol fixed bed reactor such as Figure 5 As shown, 1 g of catalyst I-b was weighed and loaded into Figure 5 In the isothermal zone of the reaction tube of the fixed bed reactor shown. Under the set reaction conditions, with N 2 The...
Embodiment 3
[0075] 0.7603g copper nitrate (Cu(NO 3 ) 2 ·3H 2 O) and 0.4542g lanthanum nitrate (La(NO) 3 ) 3 ·6H 2 O) was added to 10 ml of absolute ethanol, and after it was dissolved and mixed uniformly, 2 g of alumina carrier was added to it for immersion for 4 hours. The above mixture was dried on a rotary evaporator at 50°C and 0.09MPa for 3h, and then dried at 80°C and 0.09MPa for 2h. The dried solid matter was calcined in a muffle furnace at 450 °C for 3 h in an air atmosphere, and then calcined in a tube furnace or fixed bed reactor with 10% H 2 / N 2 Mixed gas at 500 ℃, gas air velocity 1800h-1 The catalyst I-c was obtained by reduction under conditions for 6 h. The weight content of its metal Cu is 8.4wt%, La 2 O 3 The weight content is 7.7wt%, and the rest is alumina carrier. The surface element composition and valence state of the catalyst were analyzed by XPS characterization technology, and it was proved that in the reduced catalyst, lanthanum was replaced by La. 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com