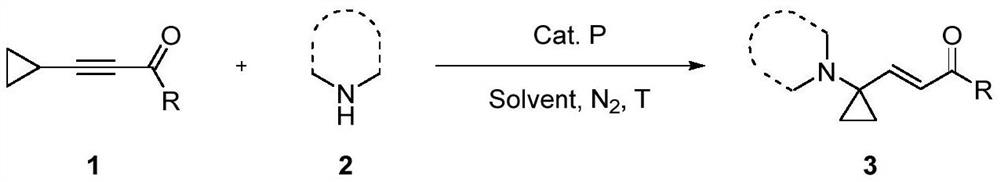
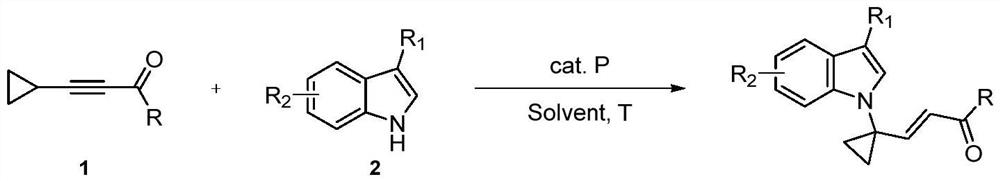
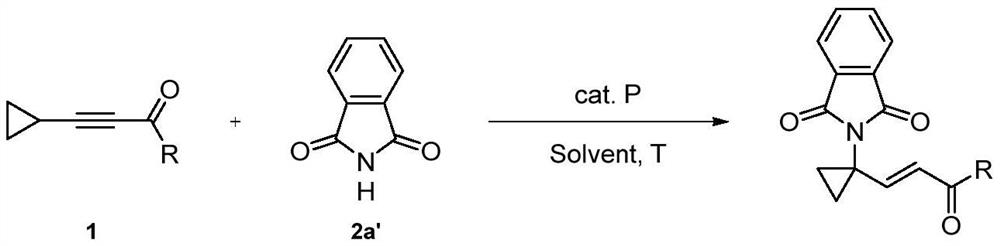
A method for constructing 1-substituted cyclopropylamine compounds by phosphine-catalyzed c-h activated amination reaction of cyclopropane
An amination reaction and compound technology, which is applied in the field of synthesis of cyclopropylamine compounds, can solve the problems of limited application scope of substrates and harsh reaction conditions, and achieves excellent regioselectivity, mild reaction conditions and environment-friendly conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Optimization of reaction conditions
[0040] Under nitrogen atmosphere, put organic phosphine catalyst (20.0 mmol% or 30.0 mmol%), cyclopropane substrate 1a (0.15 mmol), 3-nitroindole substrate 2a (0.1 mmol) into the reaction flask, use the solvent used in the reaction It was dissolved, and then the reaction flask was placed in an oil bath at room temperature or 50°C for 12 hours, the reaction solution was cooled to room temperature, 10 mL of water was added to quench the reaction, DCM (20 mL) was extracted three times, and the organic layers were combined, The solvent was removed under reduced pressure, and the pure product was obtained by column chromatography. The reaction formula was as follows:
[0041]
[0042] Table 1. Optimization of reaction conditions for amination of cyclopropane a
[0043]
Embodiment 2
[0045] Amination of Cyclopropane Substrate 1 with 3-Nitroindole Substrate 2a to Synthesize Cyclopropylamines 3
[0046] Under nitrogen atmosphere, put diphenylcyclohexylphosphine (20.0 mmol%), cyclopropane substrate 1 (0.15 mmol), 3-nitroindole substrate 2a (0.1 mmol) into the reaction flask, and use the solvent 1 for the reaction. , 4-dioxane (1.0 mL) was dissolved, and then the reaction flask was put into a 50 ℃ oil bath for 24 hours, the reaction solution was cooled to room temperature, 10 mL of water was added to quench the reaction, DCM (20 mL ) is divided into three extractions, the organic layers are combined, the solvent is removed under reduced pressure, and the column chromatography is separated to obtain a pure product, and the type of R in the cyclopropane substrate is changed to obtain different cyclopropylamine compounds 3, and the reaction formula is as follows:
[0047]
[0048] Conditions: 1(0.15mmol), 2a(0.10mmol), CyPh 2 P (20.0mol%), 1,4-dioxane (1.0mL)...
Embodiment 3
[0051] Amination of Cyclopropane Substrate 1a with Indole Substrate 2 to Synthesize Cyclopropylamines 3
[0052] Under nitrogen atmosphere, put diphenylcyclohexylphosphine or tricyclohexylphosphine (20.0 mmol%), cyclopropane substrate 1a (0.15 mmol or 0.25 mmol), and indole substrate 2 (0.1 mmol) into the reaction flask. The solvent used in the reaction was 1,4-dioxane (1.0 mL) to dissolve it, and then the reaction flask was placed in an oil bath at 50 °C for 24 hours, the reaction solution was cooled to room temperature, and 10 mL of water was added to quench the reaction , DCM (20 mL) was extracted three times, the organic layers were combined, the solvent was removed under reduced pressure, and the pure product was obtained by column chromatography, and the type of R in the indole substrate was changed to obtain different cyclopropylamine compounds 3, the reaction formula as follows:
[0053]
[0054] Conditions (except b,c Unless otherwise stated): 1a (0.15 mmol), 2 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com