Indanone quinoxaline derivative as well as preparation method and application thereof
A derivative and quinoxaline technology, applied in the field of indanonequinoxaline derivatives and their preparation, can solve the problem that the efficiency of red light materials does not reach the height of luminescent materials, and achieve easy sublimation purification, good electrochemical stability, The effect of optimizing material properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] The preparation chemical reaction formula and experimental steps of intermediate M1 are as follows:
[0065]
[0066] 1.43 g of 1H-indene-1,2,3-trione hydrate (8 mmol) and 2.13 g of 4,5-dibromobenzene-1,2-diamine (8 mmol) were dissolved in 80mL of acetic acid, and then heated to 80°C under reflux for 8 hours. After the reaction was cooled to room temperature, the resulting mixture was filtered to remove the solvent, and then the crude product was purified by silica gel column chromatography and dried under vacuum to finally obtain the product intermediate M1 with a yield of 54.8%. Product Molecular Formula: C 15 h 6 Br 2 N 2 O; molecular weight m / z: 389.88. The obtained product was subjected to an elemental analysis test, and the elemental analysis results were: C, 46.19; H, 1.55; Br, 40.97; N, 7.18; O, 4.10, and the elemental analysis results were in line with the theoretical value of the product. The product obtained was identified as M1.
[0067] The prepar...
Embodiment 1
[0075] The chemical reaction formula for preparing compound 1 in this embodiment is as follows, and its specific experimental steps are as follows:
[0076]
[0077] Intermediate M1 (1.14mmol, 445mg) and 4-(carbazol-9yl) phenylboronic acid pinacol ester (2.85mmol, 1052mg) were dissolved in K 2 CO 3 aqueous solution in a 250mL round bottom flask. After another 15 min with argon, the catalyst Pd(PPh 3 ) 4 (0.057 mmol, 66 mg), and then sparged with argon for 10 minutes. The reaction was then heated to 85°C and stirred vigorously for 12 hours. After cooling the reaction system to room temperature, the solvent was removed by vacuum rotary evaporator, and the mixture was extracted 3 times with dichloromethane (DCM). The collected organic phase was further washed three times with deionized water, and finally the organic phase was washed with anhydrous magnesium sulfate (MgSO 4 )dry. The next step was purified by silica gel column chromatography to finally obtain the product...
Embodiment 2
[0079] The chemical reaction formula for preparing compound 2 in this embodiment is as follows, and its specific experimental steps are as follows:
[0080]
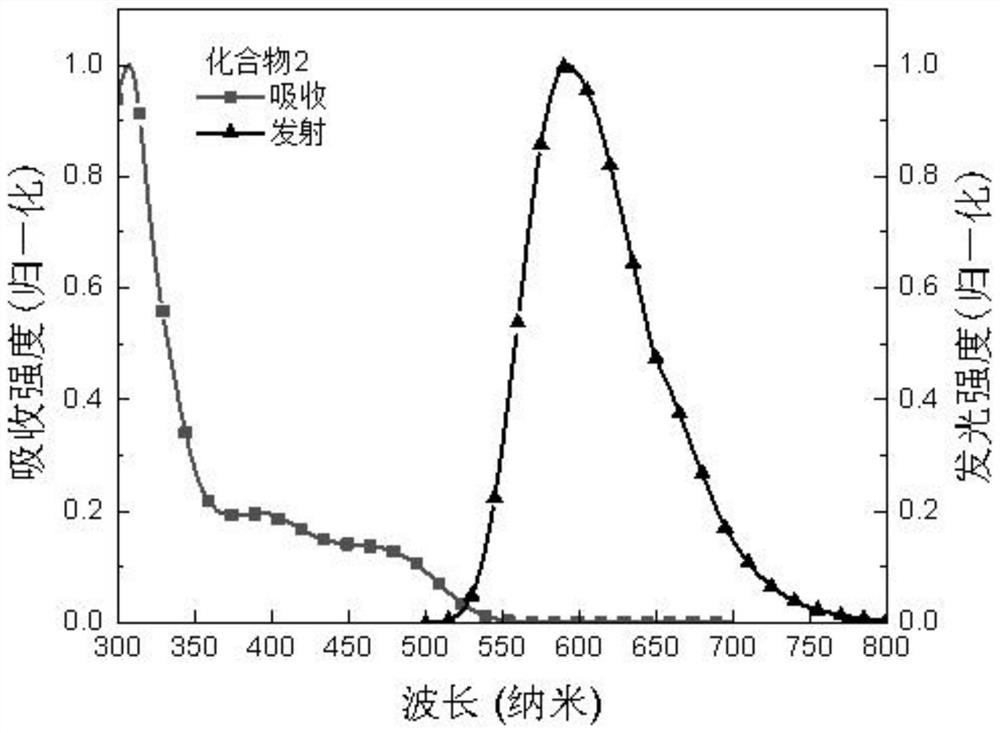
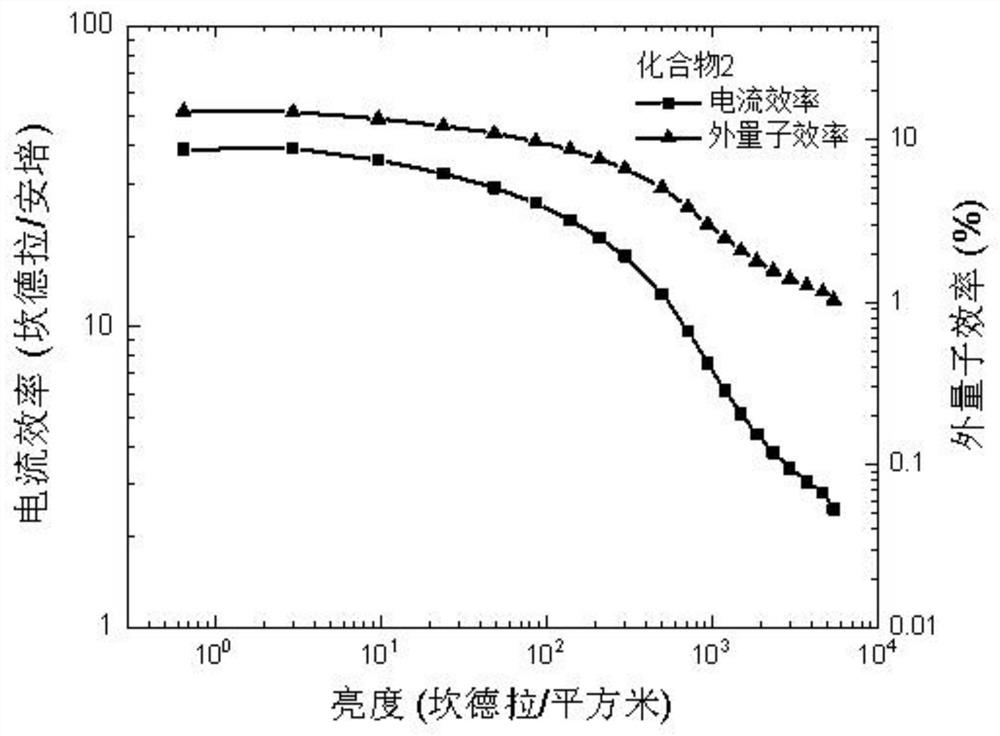
[0081] Compared with Example 1, the difference is that 4-(carbazol-9 base) phenylboronic acid pinacol ester is replaced with equivalent triphenylamine-4-boric acid pinacol ester, and other raw materials and steps are the same as Example 1, the product compound 2 was finally obtained with a yield of 76%. Product Molecular Formula: C 51 h 34 N 4 O; molecular weight m / z: 718.27. The obtained product was subjected to an elemental analysis test, and the elemental analysis results were: C, 85.21; H, 4.77; N, 7.79; O, 2.23, and the elemental analysis results were in line with the theoretical value of the product. The product obtained was identified as compound 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap