Compositions and methods for genetic modification and targeting
A genetic modification, mammalian technology, applied in the field of organs and non-human mammals, cells, and tissues, which can solve the problems of expensive and time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0199] Example 1. Genetically modified pigs exhibit enhanced resistance to ASFV
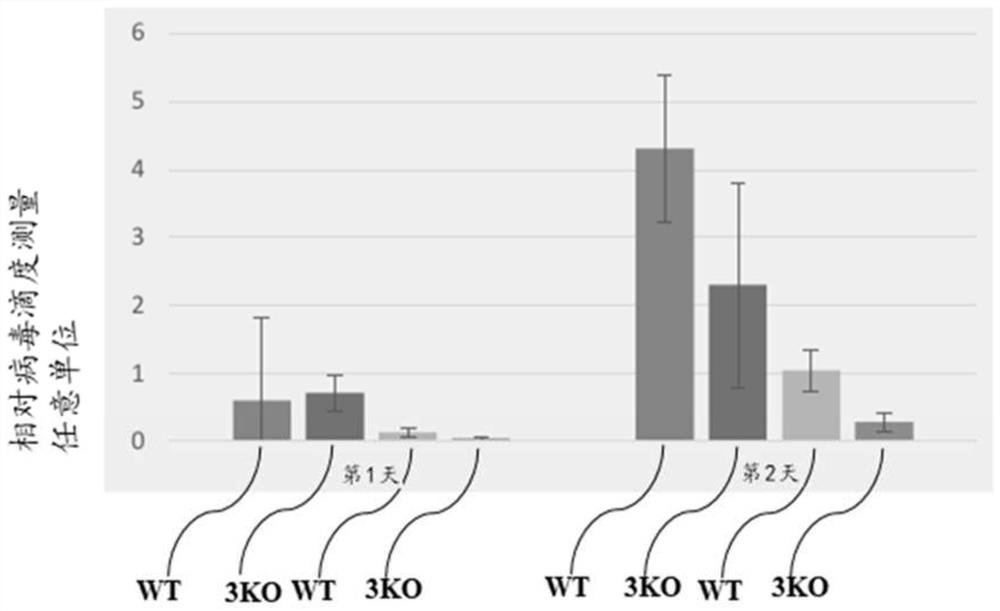
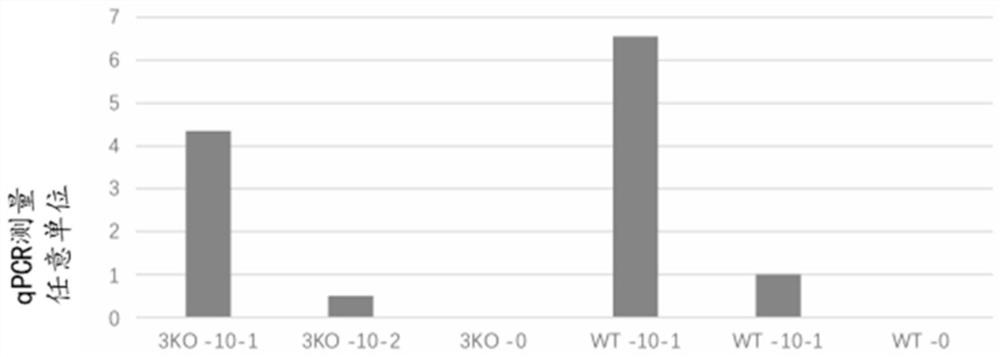
[0200] Genetically modified pigs comprising knockouts of three endogenous genes (CMAH, GGTA1 and B4GALNT2) were examined for resistance to ASFV. Porcine alveolar macrophages were isolated from genetically modified triple knockout pigs (3KO group) and unmodified controls (WT group). Porcine alveolar macrophages were seeded on polylysine-coated plates and challenged with ASFV infection at various dilutions. After ASFV infection, supernatants and cell pellets were collected and examined for ASFV viral replication. Figure 1A Shown in the use of reduced virus titer (10 -2 ) on day 2, ASFV replication was significantly reduced in the 3KO group compared to the WT control group. ASFV virus copy numbers in supernatants and alveolar macrophage pellets were measured by qPCR. Figure 1B It was shown that the supernatant obtained from the 3KO group exhibited lower ASFV virus copy number within 72 hours. ...
Embodiment 2
[0201] Example 2. Genetic modification of endogenous RELA gene
[0202] To enhance resistance to viral infection by ASFV, the endogenous RELA gene was genetically modified in pigs. Figure 2A The top panel of , shows the locus (exon 13) into which the modified RELA allele was introduced by CRISPR / Cas9-directed homologous recombination. The modified RELA allele corresponds to the ASFV-resistant warthog RELA allele and is flanked at the 5' and 3' ends by 1.5 kb homology arms (e.g. the first 1.5 kb homology arm followed by the modified allele gene, followed by a second 1.5 kb homology arm). Figure 2A The lower panel of , shows the guide nucleic acid (gRNA) targeting and binding sites for gRNA 1 and gRNA 2. Change the gRNA target site in the donor plasmid to a synonymous codon. Figure 2B Screening of candidate clones with biallelic modified RELA knock-ins is shown. The PCR product shown in the agarose gel corresponds to the presence of the knock-in RELA allele. Figure 2C S...
Embodiment 3
[0203] Example 3. Targeting multiple regions of the ASFV viral genome for cleavage and degradation
[0204] To effectively combat viral infections, guide nucleic acids (gRNAs) were designed for multiple targeting of viral genomes. Such multiplexing reduces the likelihood of the viral genome escaping cleavage due to mutations in the viral genome. The gRNA is also designed to avoid targeting and binding of the host cell's genomic sequence. Other design criteria included targeting ASFV coding and conserved regions. The gRNA was designed to be recognized by Cas9 from Streptococcus pyogenes, which recognized the PAM sequence 5'-NGG-3' (where "N" can be any nucleotide base) as part of the gRNA. Targets with a GC content between 20% and 80% have high priority; avoid homopolymers of U nucleotides; and avoid off-target cleavage with up to two mismatches with the host cell's genome.
[0205] gRNAs were designed to target the sequences shown in Table 3. SEQ ID NO: 6 is the nucleic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com