Ratio fluorescence immunochromatography detection test strip and detection method thereof
An immunochromatographic detection and ratiometric fluorescence technology, which is applied in the field of medical testing, can solve the problems of reduced sensitivity, complex chromatography process of test strips, and inability to accurately and truly reflect the substances to be tested.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
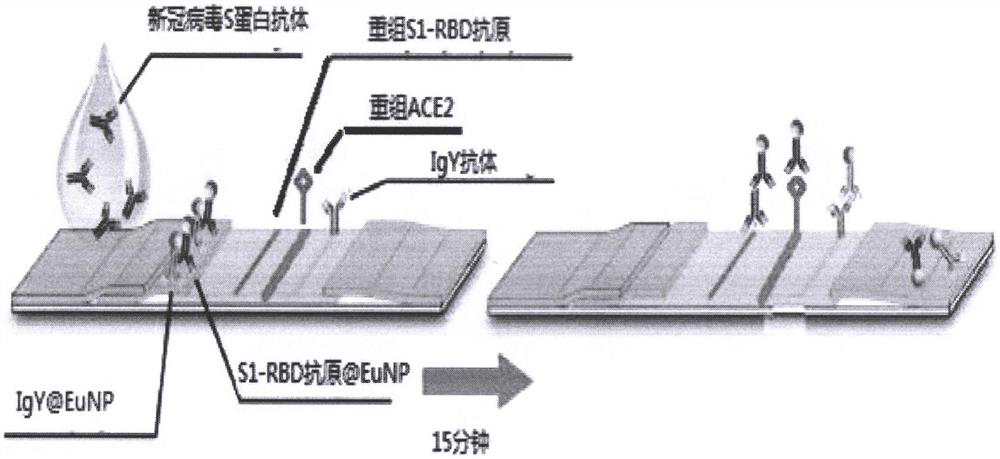
[0044] The preparation of embodiment 1 immunochromatography test strip
[0045] Preparation of recombinant new coronavirus S1-RBD antigen solution: Ultrafiltration of recombinant new coronavirus S1-RBD antigen with borate buffer solution to obtain recombinant new coronavirus S1-RBD antigen solution; adjust the concentration of recombinant new coronavirus S1-RBD antigen solution to 1.0mg / mL.
[0046] Prepare angiotensin-converting enzyme 2 (ACE2) recombinant protein solution: use borate buffer to obtain angiotensin-converting enzyme 2 (ACE2) recombinant protein solution by ultrafiltration of recombinant angiotensin-converting enzyme 2 (ACE2) recombinant protein; The concentration of angiotensin-converting enzyme 2 (ACE2) recombinant protein solution was adjusted to 2.0 mg / mL.
[0047] Spray the recombinant new coronavirus S1-RBD antigen solution and angiotensin converting enzyme 2 (ACE2) recombinant protein solution on the nitrocellulose membrane with a gold spray machine to ...
Embodiment 2
[0049] Embodiment 2 detects the sample to be tested
[0050] (1) Preparation of sample diluent
[0051] In the borate buffer solution with pH 8.0-8.5, add Tween 20 at a final concentration of 1% (v / v), 0.9% NaCl at a final concentration and proclin300 at a final concentration of 0.03% at a final concentration of 0.2 % (v / v) Biosaccharide Gum-1.
[0052] (2) Sample preparation
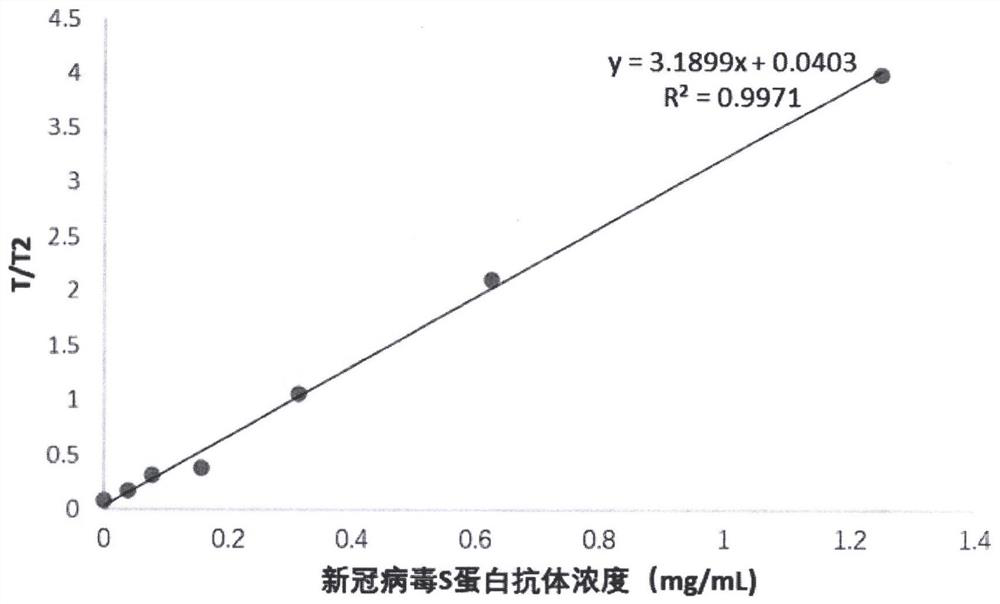
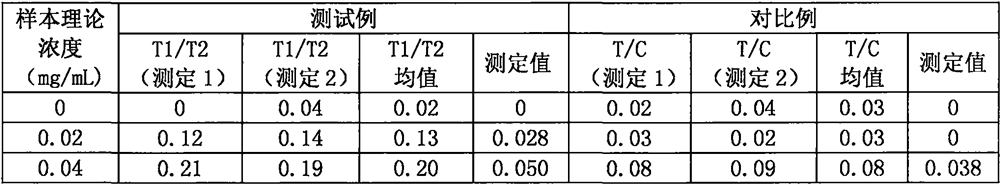
[0053] The anti-new coronavirus RBD single domain antibody was serially diluted with normal human serum in 2018 physical examination, and the concentrations were 0mg / mL, 0.039mg / mL, 0.078mg / mL, 0.156mg / mL, 0.313mg / mL, 0.625mg / mL, respectively. mL, 1.25mg / mL series of anti-new coronavirus RBD single domain antibody calibration solutions.
[0054] (3) Drawing of calibration curve
[0055] Take the test strip, equilibrate at room temperature for 10 minutes, open the package and take out the test strip; take 50 μL of the anti-new coronavirus RBD single domain antibody solution calibration solution and 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com