Method for screening point mutation BIRC5 antigen epitope peptide
An antigen epitope and epitope peptide technology, applied in the field of molecular biology, can solve the problems of effective activation and expansion of T cells, low binding force, etc., achieve high accuracy, simple experimental process, and improve the effect of clinical trials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0122] Bioinformatics analysis of embodiment 1 BIRC5 epitope
[0123] (1) NetMHCpan 4.1 online prediction system:
[0124] Log in to the website http: / / www.cbs.dtu.dk / services / NetMHCpan / , in "SUBMISSION", select "Fasta" for the "INPUT TYPE" item, enter the full amino acid sequence of BIRC5, and select "9mer" for the "PEPTIDE LENGTH" item peptides", select "HLA-A*02:01" in the "SELECT SPECIES / LOCI" project, and click "Submit" to run the remote prediction program.
[0125] The results were sorted according to the %Rank_EL score. The higher the %Rank_EL score, the weaker the binding ability of the epitope peptide to MHC molecules. We selected epitope peptides with 21<%Rank_EL score<30 for subsequent analysis. The list of epitope peptides with 21<%Rank_EL score<30 is shown in Table 1.
[0126] Table 1 NetMHCpan 4.1 prediction results of BIRC5 antigen 9 amino acid CTL epitope
[0127]
[0128] (2) SYFPEITHI online prediction system:
[0129] Login URL http: / / www.syfpeithi....
Embodiment 2
[0142] Example 2 Detection of the actual binding ability of the epitope peptide and the HLA-A2 molecule
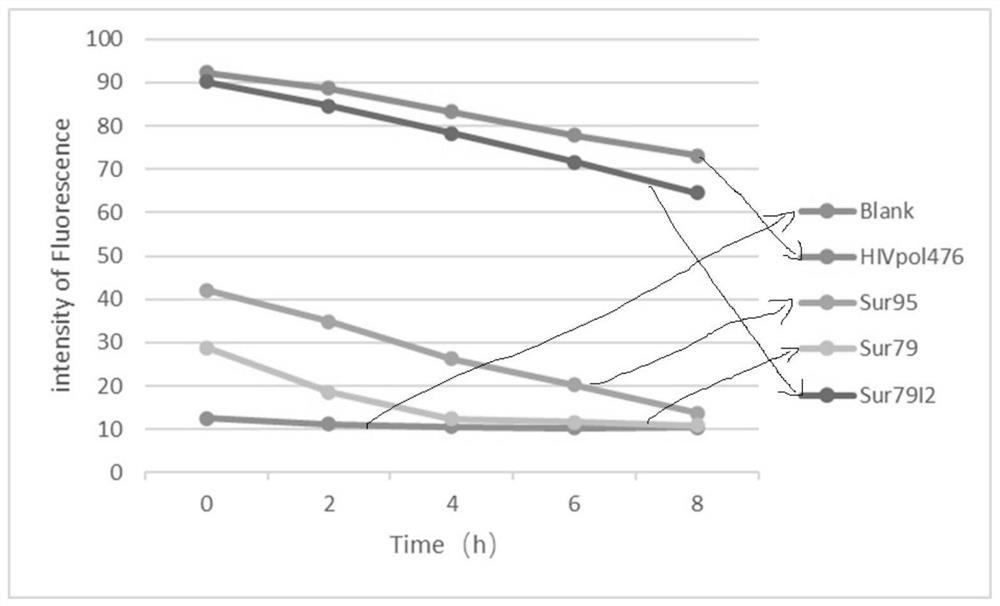
[0143] Artificially synthesized candidate peptides include Sur79, Sur79I2 (mutant peptide), HIVpol 476 (negative peptide with high affinity with HLA-A2 molecules), Sur95 (positive peptide), a total of 4 groups.
[0144] (1) Detection of affinity between candidate epitope peptides and HLA-A2 molecules
[0145] Step Ⅰ) Take 1×10 for each group 6 / mL T2 cells were cultured overnight in DMEM medium containing 20% FBS. After collecting the cells, they were washed twice with serum-free AIM-V medium and then resuspended and seeded in 24-well cell culture plates. Added 10 μM of different species The synthetic polypeptide and 3 μg / mL β2-MG were incubated at 37°C for 18 hours, and washed twice with PBS containing 3% FBS.
[0146] Step II) Resuspend the mixture obtained above in AIM-V medium for 2 hours at 37°C, collect cells, add FITC-labeled anti-HLA-A2 flow cytometry antibody...
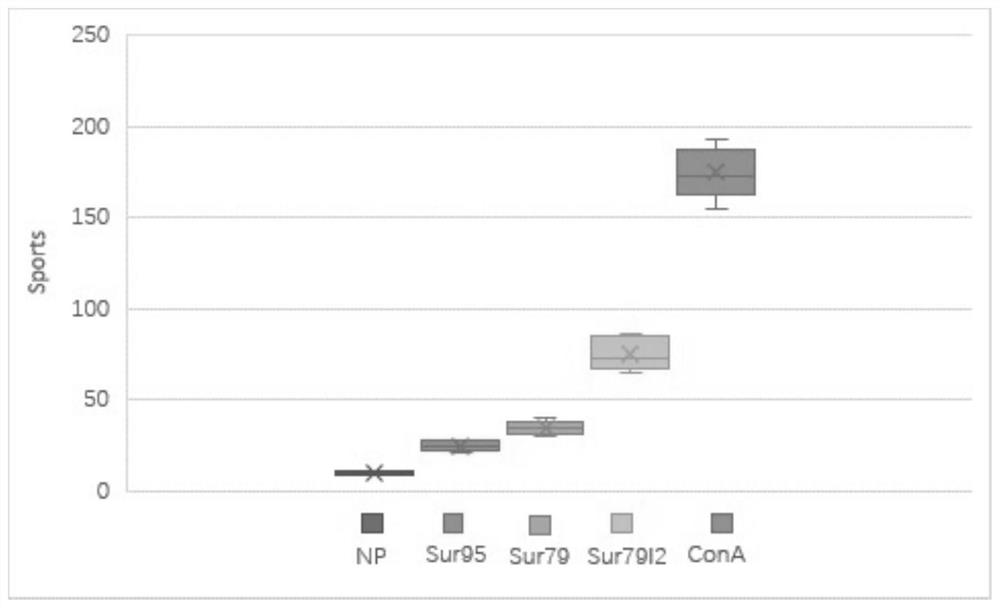
Embodiment 3
[0157] Example 3 Establishment of CTLs clones induced by DC and mutant peptides in vitro
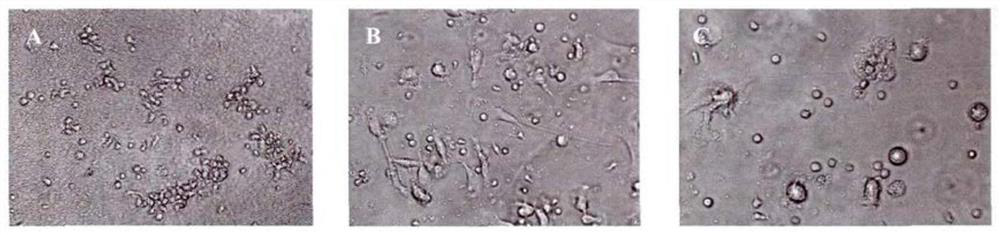
[0158] (1) DC differentiation induced in vitro
[0159] Firstly, prepare the special culture medium for induction and differentiation of DC in vitro: add 10% FBS, 150 μg / mL rhGM-CSF and 50 μg / mL rhIL-4 to RPMI-1640 liquid medium, mix well, and store at 4 °C for later use.
[0160] Collect the adherent mononuclear cells obtained before, and use DC-specific culture medium to divide the cells into 1×10 6 / mL density and seeded in 48-well plate, 0.5ml / well. The cells were cultured for 6 days at 37°C in an incubator with 5% CO2 saturated humidity. On the third day and the fifth day, half of the medium was changed. On the 8th day, 400 ng / mL rhTNF-α (10 ng / mL) was added to treat for 24 hours to stimulate DC maturation, and the obtained DCs were mature DCs (mDCs) for future use. The DC growth was observed with an inverted phase-contrast microscope, and the surface molecular markers of mature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com