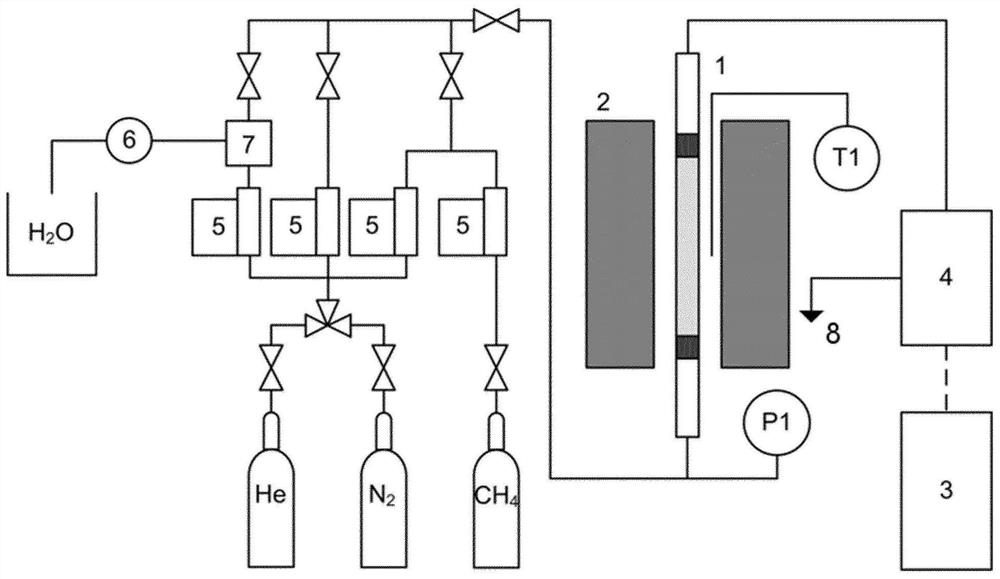
Redox preparation process of an oxygen carrier for a chemical looping process
A technology of oxygen carrier and chemical chain, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of oxygen carrier materials, energy density, and expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] by containing 20 vol% H at a temperature of 850°C 2 The oxygen carrier material was reduced overnight in He flow (100 seem) to obtain pre-reduced oxygen carrier material.
[0109] 0.76g CuCl 2 .2H 2 O dissolved in 1.1ml H 2 O, thus obtaining 4.05M Cu 2+ solution. 0.050 g of this solution was contacted with 0.5072 g of pre-reduced oxygen carrier (OC) material. In the case of a quantitative reaction, 0.013 g of Cu could be deposited. The observed final Fe:Cu molar ratio was 36.1:1.
Embodiment 2
[0111] 0.0241g CuCl 2 .2H 2 O dissolved in 0.4993g H 2 O, thus obtaining 0.283M Cu 2+ solution.
[0112] 0.30 g of this solution was contacted with 1 g of pre-reduced oxygen carrier material obtained by heating at 900° C. 2 Obtained by overnight reduction in He (100 sccm). 0.0054 g Cu could be deposited in case of quantitative reaction. The Fe:Cu molar ratio in the final product obtained was observed to be 87:1.
Embodiment 3
[0114] 0.4614g CuCl 2 .2H 2 O dissolved in 0.5058g H 2 O, thus obtaining 5.35M in H 2 solution in O. 0.30 g of this solution was contacted overnight with 1 g of pre-reduced oxygen carrier material produced by heating at 900° C. 2 obtained by reducing the oxygen carrier material in the presence of flowing He (100 sccm).
[0115] In case of quantitative reaction, 0.102 g Cu can be deposited on the oxygen carrier material. A final Fe:Cu molar ratio of 3.7:1 was obtained
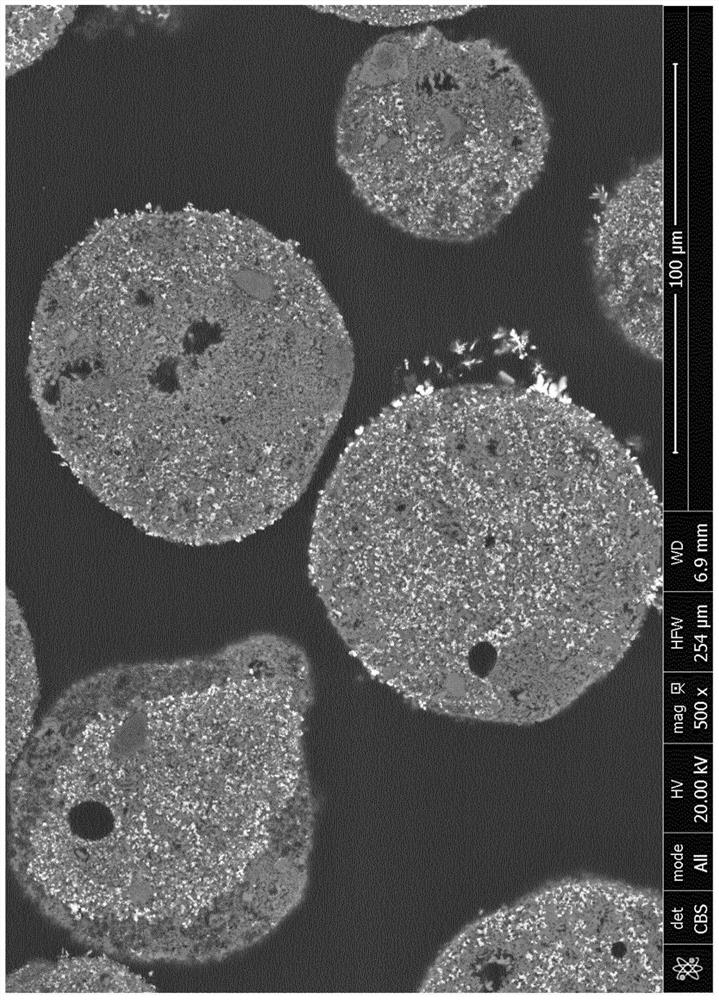
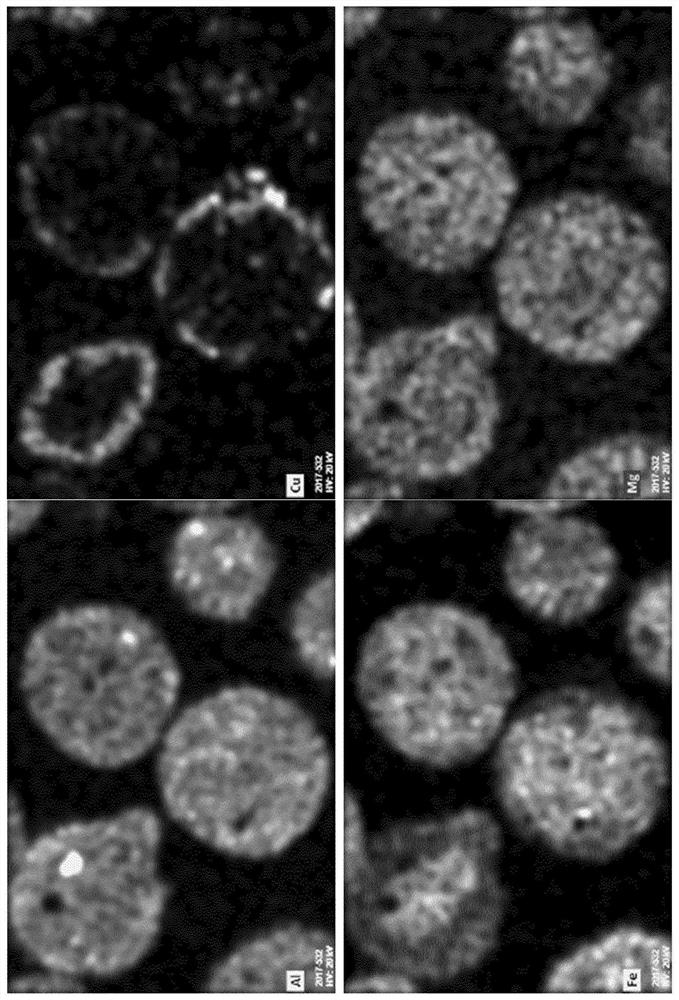
[0116] SEM & Optical Microscopy
[0117] On polished cross-sections of embedded particles inside epoxy resin, the concentration of oxygen carriers was studied by optical microscopy (SteREO imager, ZEISS) and by scanning electron microscopy using a FEI NOVA Nanosem 450 with an energy dispersive spectrometer (EDS) system. microstructure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com