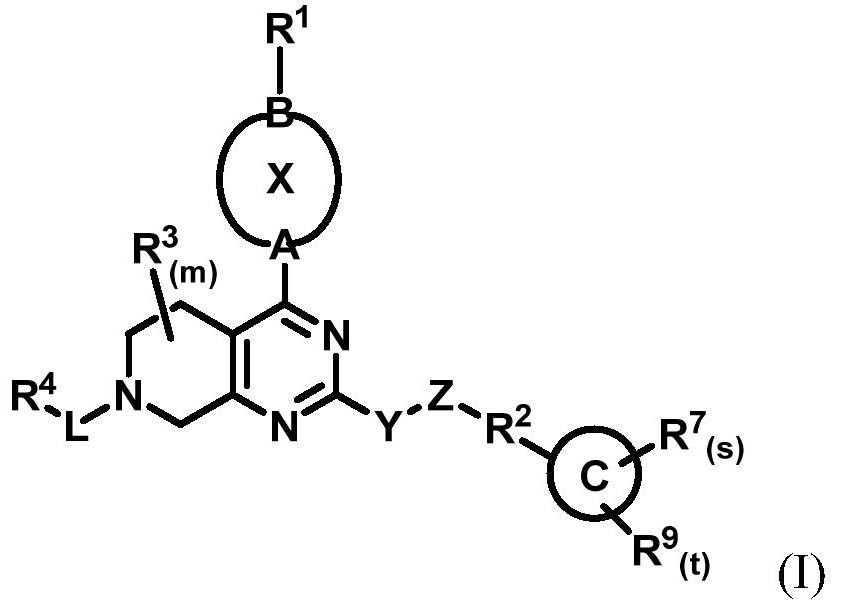
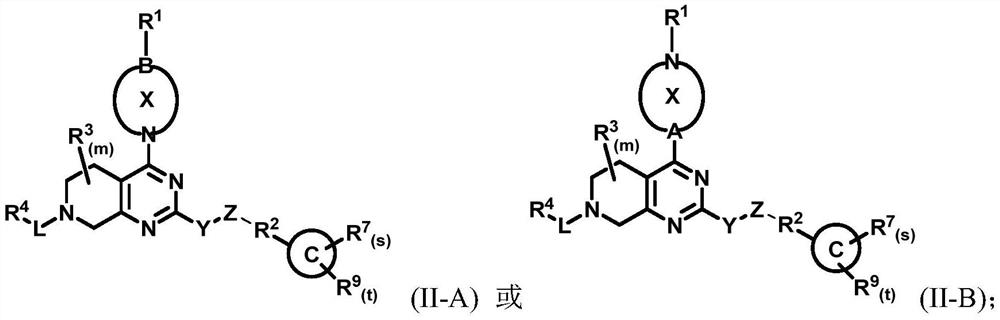
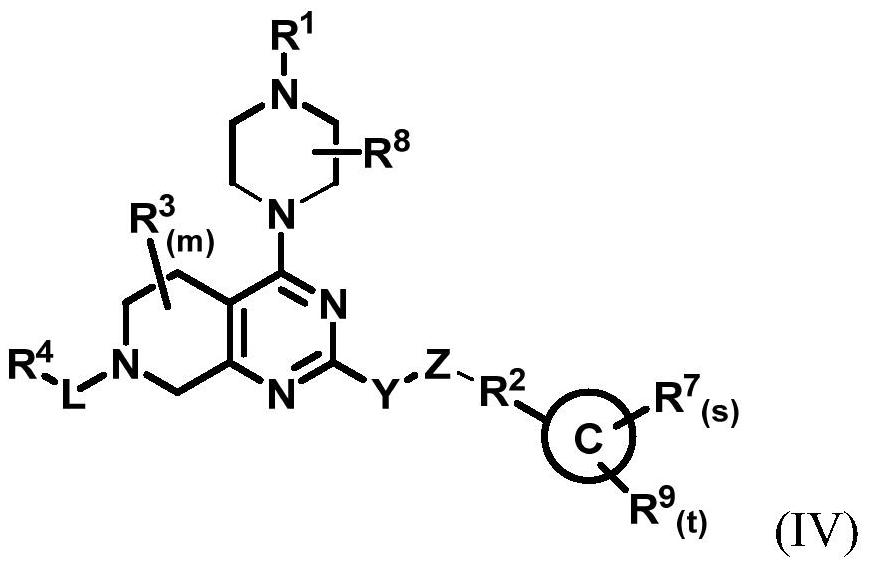
Alkoxy alkyl substituted heterocyclic group inhibitor as well as preparation method and application thereof
The technology of a heterocyclic group and an alkoxy group is applied in the field of alkoxyalkyl substituted heterocyclic group inhibitors and their preparation, which can solve the problem of no drug approval and marketing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
preparation example Construction
[0249] The present invention also provides a preparation method of a pharmaceutical composition, comprising the steps of: mixing a pharmaceutically acceptable carrier with the compound of general formula (I) or its crystal form, pharmaceutically acceptable salt, hydrate or The solvates are mixed to form a pharmaceutical composition.
[0250] The present invention also provides a treatment method, which includes the steps of: administering the compound of general formula (I) described in the present invention, or its crystal form, pharmaceutically acceptable salt, hydrate or solvate to the subject in need of treatment , or administer the pharmaceutical composition of the present invention for selectively inhibiting KRAS G12C .
[0251] The present invention has the following main advantages:
[0252] (1) The compound is to KRAS G12C Has a good selective inhibitory effect;
[0253] (2) The compound has better pharmacodynamics, pharmacokinetic properties and lower toxic and s...
Embodiment 1
[0297] Example 1 (S)-2-(4-(7-(8-chloronaphthalen-1-yl)-2-((3-(methoxymethyl)-1-methylcyclobutylamine-3- base)methoxy)-5,6,7,8-tetrahydropyridin[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile preparation
[0298]
[0299] The first step: (S)-4-(7-(8-chloronaphthalen-1-yl)-2-((3-(methoxymethyl)-1-methylcyclobutylamine-3-yl) Preparation of tert-butyl methoxy)-5,6,7,8-tetrahydropyridin[3,4-d]pyrimidin-4-yl)-2-(cyanomethyl)piperazine-1-carboxylate
[0300] (S)-2-(cyanomethyl)-4-(7-(8-chloronaphthalen-1-yl)-2-(methylsulfoxide)-5,6,7,8-tetrahydro Pyridin[3,4-d]pyrimidin-4-yl)piperazine-1-carboxylic acid tert-butyl ester (74mg, 0.128mmol) was added to the reaction flask, followed by adding toluene (0.8mL), (3-methoxy Methyl-1-methyl-azetidin-3-yl)-methanol (37 mg, 0.256 mmol) and sodium tert-butoxide (37 mg, 0.384 mmol). The reaction solution was stirred under ice-water bath for 0.5 h, then water (50 mL) was added, and then extracted with ethyl acetate (3...
Embodiment 2
[0309] Example 2 (S)-2-(4-(7-(8-chloro-7-fluoronaphthalen-1-yl)-2-((3-(methoxymethyl)-1-methylcyclobutane Amine-3-yl)methoxy)-5,6,7,8-tetrahydropyridin[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazine-2- base) acetonitrile
[0310]
[0311] LCMS:m / z 652(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com