Application of metformin in preparation of medicine for treating hand-foot-and-mouth disease
A technology for metformin and hand, foot and mouth disease, which is applied in the directions of pharmaceutical formulations, antiviral agents, and medical preparations containing active ingredients, and can solve problems such as the lack of metformin application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
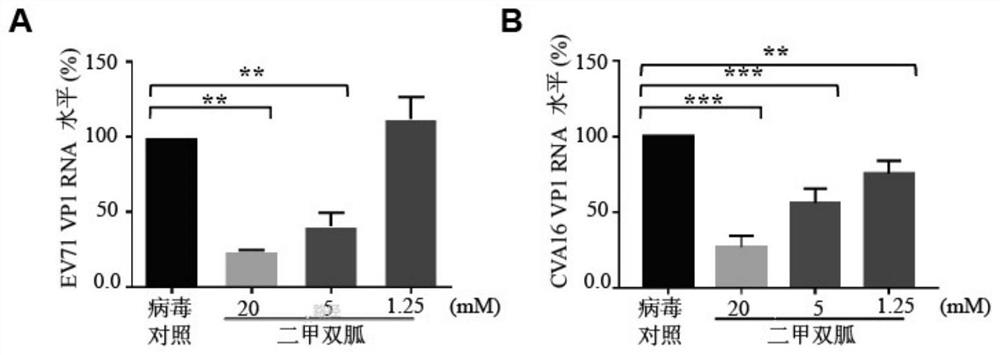
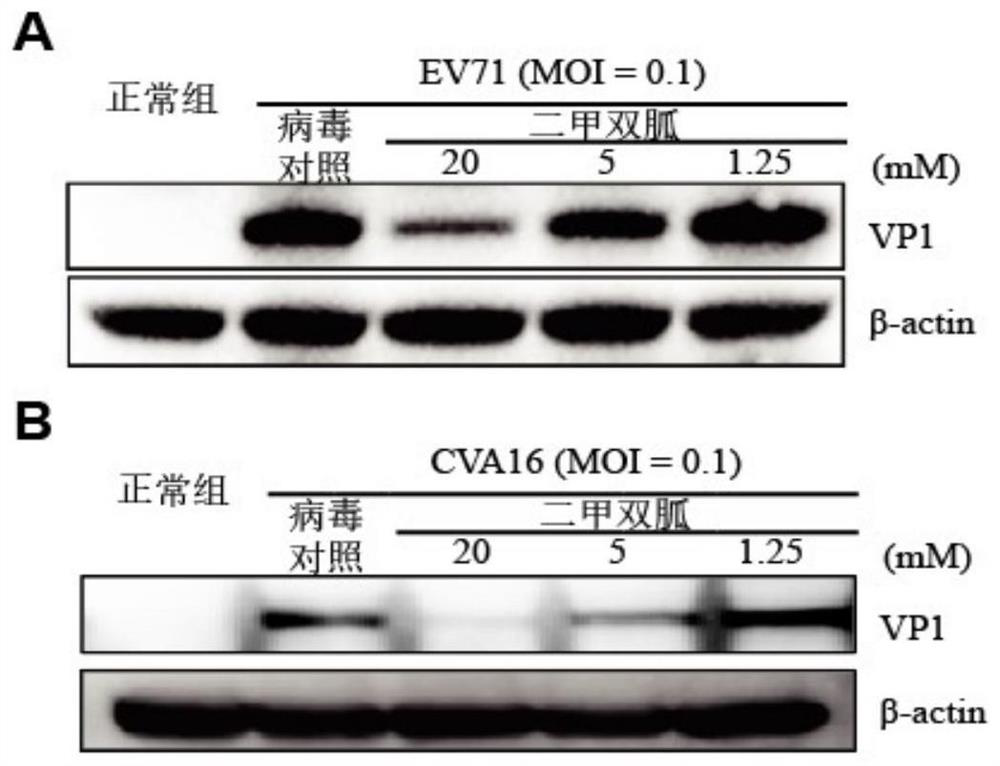
[0036] Example 1 Inhibitory Effect of Metformin on EV71 and CVA16 Replication
[0037] 1. Cell Culture
[0038] Culture medium for subculture of HCT-8 cells: DMEM (Gibco) culture medium containing 10% fetal bovine serum (Gbico), penicillin and streptomycin double antibody 100 U / ml (Gibco).
[0039] The culture medium used for HCT-8 cell dilution virus: DMEM culture medium containing penicillin and streptomycin double antibody 100U / ml (Gibco).
[0040] The culture medium used for HCT-8 cell dilution drug: DMEM culture medium containing 2% fetal bovine serum (Gbico), penicillin and streptomycin double antibody 100U / ml (Gibco).
[0041]When the confluence of HCT-8 cells reaches 90%, add 0.25% trypsin-EDTA (Gibco) to the culture flask, digest at 37°C for 2 minutes, discard the trypsin, add complete culture medium to blow off, passage at 1:3, 1- Passage once every 2 days.
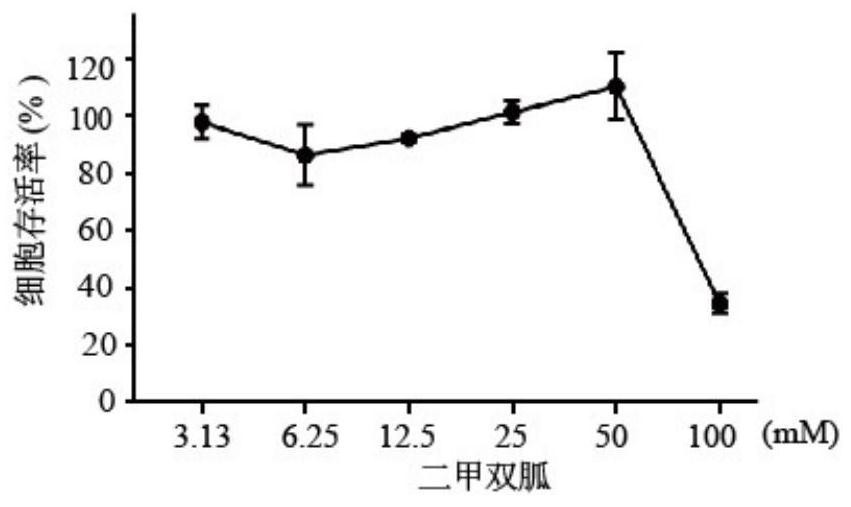
[0042] 2. Cytotoxicity detection
[0043] HCT-8 cells were seeded in 96-well plates, 3×10 4 pc / well, 37℃...
Embodiment 2 2
[0072] Example 2 The inactivation effect of metformin on EV71 and CVA16
[0073] Vero cells were seeded in 6-well plates, 9×10 5 pc / well, 37℃, 5%CO2 After 24 hours, the medium in the well plate was discarded and added at 37°C, 5% CO 2 Cells were infected with EV71 or CVA16 for 1 h after co-incubating with metformin or DMSO for 1 h. After 1 hour, the virus liquid was discarded and the culture medium used for diluting the drug was added, and the virus liquid was incubated at 37°C and 5% CO. 2 CPE was observed and recorded after being cultured in medium for 72 hours, and half of the tissue cell infection amount of the virus (50% tissue culture infectious doses, TCID) was calculated according to the CPE results. 50 ).
[0074] from Figure 5 It can be seen that metformin has no direct inactivation effect on EV71 and CVA16.
Embodiment 3 2
[0075] Example 3 Effect of metformin on EV71 and CVA16 receptor SCARB2 protein content
[0076] HCT-8 cells were seeded in 6-well plates, 9×10 5 pc / well, 37℃, 5%CO 2 After 24 hours, the medium in the well plate was discarded, and different concentrations of metformin were added. A normal control group (the culture fluid used for diluting the drug) and a metformin drug group (20mM, 5mM and 1.25mM) were set. 24 hours after the addition of the drug, the total cell protein was extracted and detected by western blot, which was the same as in Example 1, wherein the SCARB2 antibody was a product of Abcam.
[0077] from Image 6 As can be seen, metformin dose-dependently downregulated the protein content of EV71 and CVA16 receptor SCARB2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com