Anti-PD-1 monoclonal antibody liquid preparation
A monoclonal antibody, liquid preparation technology, applied in the direction of antibodies, antibody medical components, anti-tumor drugs, etc., can solve the problems of poor stability of monoclonal antibodies, and achieve the effect of improving stability and excellent long-term stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The influence of embodiment 1pH on formula
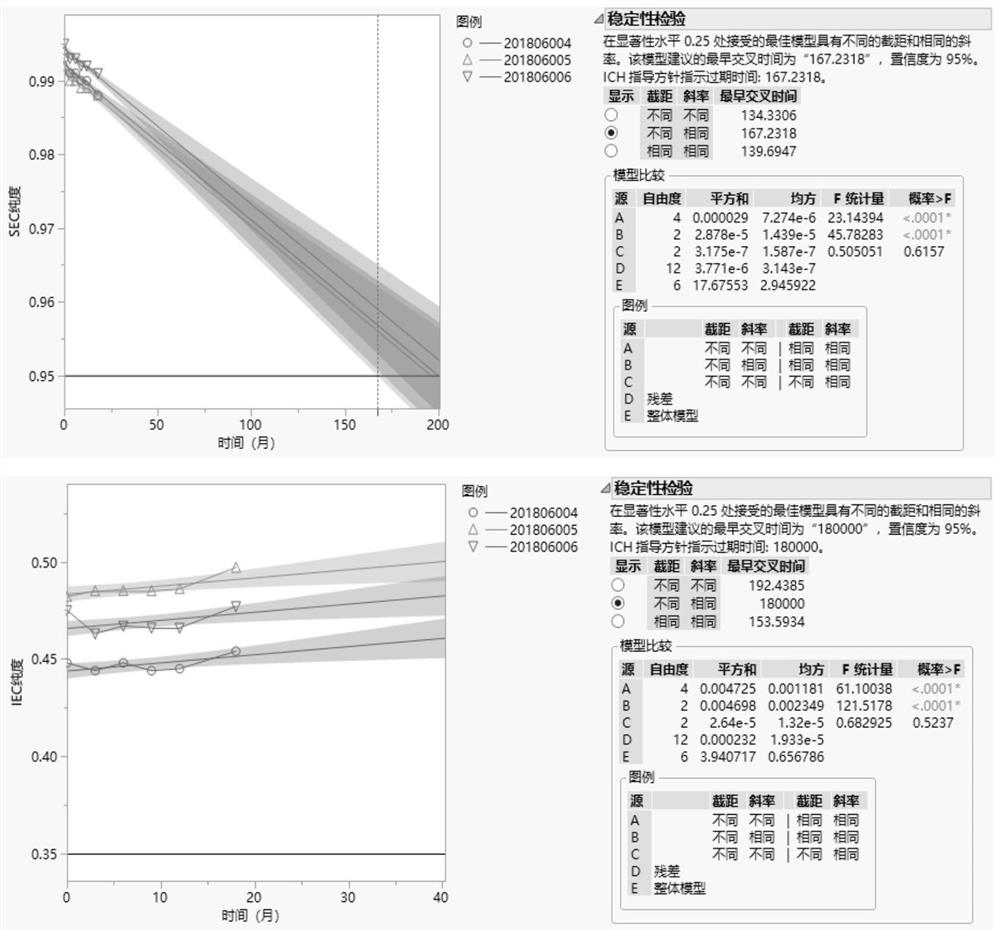
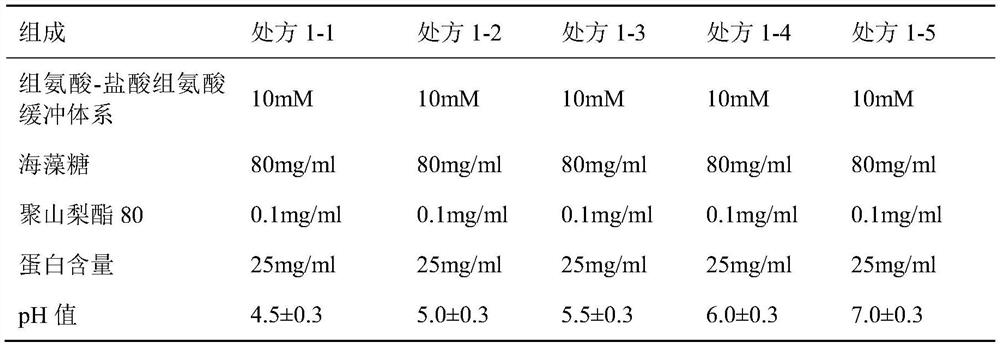
[0049] Prepare 5 kinds of buffer solutions with different pH values in Table 1, and prepare the protein and auxiliary materials to obtain the prescription solutions in the following table. After being sterilized and filtered by a 0.22μm filter membrane, 1ml / bottle was divided into 2ml vials, stoppered and capped to obtain candidate prescription samples, and placed in a stability test box at 40±2°C. Sampling and investigation at 2 weeks, 4 weeks, 6 weeks, and 8 weeks, and the inspection indicators are SEC purity and IEC purity.
[0050] Table 1 pH investigation
[0051]
[0052] The results are shown in Table 2. The results show that with the increase of the pH value, the decrease rate of the SEC purity of the protein in each prescription solution is gradually accelerated, and the pH range of 4.5-6.0 is better. The change trend of IEC purity shows that prescription 1-2, prescription 1-3 and prescription 1-4 are better t...
Embodiment 2
[0055] The impact of embodiment 2 buffer system on formula
[0056] Prepare 4 different buffer system solutions in Table 3, and prepare the protein and excipients to obtain the target prescription solution. After being sterilized and filtered by a 0.22μm filter membrane, 1ml / bottle was divided into 2ml vials, stoppered and capped to obtain candidate prescription samples, and placed in a stability test box at 40±2°C. Sampling and investigation at 2 weeks, 4 weeks, 6 weeks, and 8 weeks, and the inspection indicators are SEC purity and IEC purity.
[0057] Table 3 Buffer system screening prescription information
[0058]
[0059] Investigating the stability of each formulation under the condition of 40±2℃, the change trend of SEC purity and IEC purity showed that prescription 2-1 and prescription 2-2 were better than prescription 2-3 and prescription 2-4; There were no significant differences between prescriptions. It can be seen that the acetic acid buffer system and the h...
Embodiment 3
[0063] Embodiment 3 albumen protectant and surfactant affect formula
[0064] Prepare the histidine-histidine hydrochloride buffer system in Table 5, and prepare the protein and excipients to obtain the target prescription solution. After being sterilized and filtered by a 0.22μm filter membrane, 1ml / bottle was divided into 2ml vials, stoppered and capped to obtain candidate prescription samples, and placed in a stability test box at 40±2°C. Sampling and investigation at 2 weeks, 4 weeks, and 8 weeks, and the inspection indicators are SEC purity, IEC purity, and insoluble particles.
[0065] Table 5 Screening Prescription Information of Protein Protectants and Surfactants
[0066]
[0067] The stability, insoluble particles and SEC purity of each formulation were investigated under the condition of 40±2°C. The results further showed that the addition of sugar protective agent and polysorbate in the formulation could effectively improve the stability; there was no significa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com