Synthesis method of pentafluorophenyl dioxazolone
A technology of pentafluorophenyl azlactone and its synthesis method, which is applied in the field of synthesis of pentafluorophenyl azlactone, and can solve problems such as poor purity, low production capacity, and imperfect pentafluorophenyl azlactone , to achieve the effect of simple reaction process, mild reaction process and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The synthetic method of pentafluorophenyl dioxazolone, its synthetic steps are as follows:
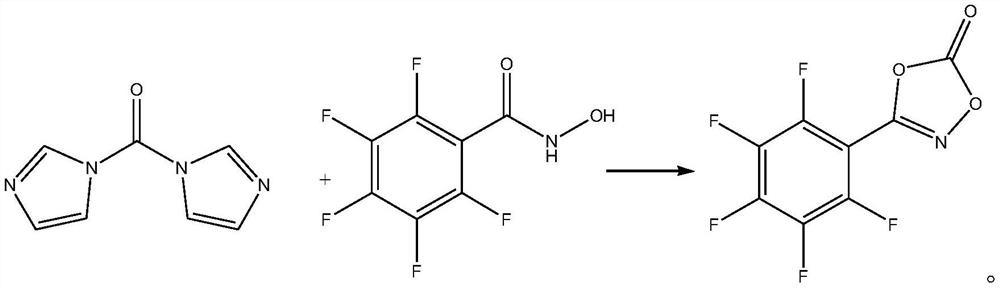
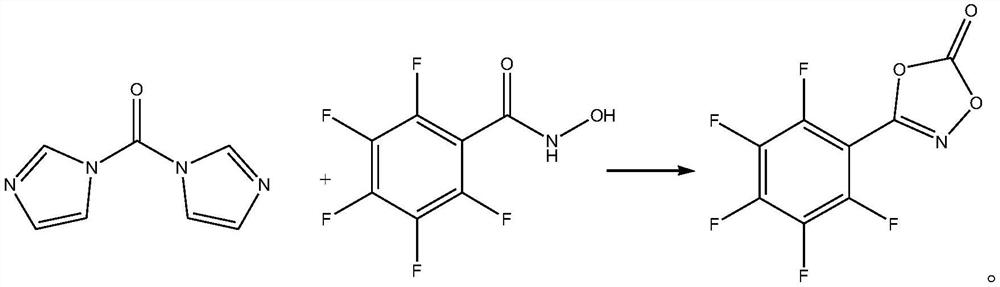
[0020] Take 36.79g (0.162mol) of 2,3,4,5,6-pentafluoro-N-hydroxyl-benzamide and dissolve it in 400mL of freshly distilled dichloromethane, then add 16.2g (0.1mol) of N,N '-Carbonyldiimidazole, carry out cyclization reaction at room temperature for 15min, TLC monitors until the reaction of N,N'-carbonyldiimidazole is complete, add 810mL of 1mol / L hydrochloric acid aqueous solution and stir for neutralization reaction, then add 500mL of ethyl acetate to extract , phase separation, the resulting ethyl acetate phase was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain 8.79 g of 3-(2,3,4,5,6-pentafluorophenyl)-1,4,2-bis Oxazol-5-one, yield 33.41%, purity 99.6%, specific chemical reaction formula is as follows:
Embodiment 2
[0022] The synthetic method of pentafluorophenyl dioxazolone, its synthetic steps are as follows
[0023] Take 34.06g (0.15mol) of 2,3,4,5,6-pentafluoro-N-hydroxy-benzamide and dissolve it in 324mL of freshly distilled dichloromethane, then add 16.2g (0.1mol) of N,N '-Carbonyldiimidazole, carry out cyclization reaction at room temperature for 30min, TLC monitors until the reaction of N,N'-carbonyldiimidazole is complete, add 405mL aqueous hydrochloric acid solution with a concentration of 2mol / L and stir for neutralization reaction, then add 400mL ethyl acetate to extract , phase separation, the resulting ethyl acetate phase was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain 8.81 g of 3-(2,3,4,5,6-pentafluorophenyl)-1,4,2-bis Oxazol-5-one, yield 33.49%, purity 99.5%.
Embodiment 3
[0025] The synthetic method of pentafluorophenyl dioxazolone, its synthetic steps are as follows:
[0026] Take 40.88g (0.18mol) of 2,3,4,5,6-pentafluoro-N-hydroxyl-benzamide and dissolve it in 390mL of freshly distilled dichloromethane, then add 16.2g (0.1mol) of N,N '-Carbonyldiimidazole, carry out cyclization reaction at room temperature for 10min, TLC monitors until the reaction of N,N'-carbonyldiimidazole is complete, add 650mL of 1.5mol / L hydrochloric acid aqueous solution and stir for neutralization reaction, then add 500mL of ethyl acetate Extraction and phase separation, the resulting ethyl acetate phase was dried over anhydrous magnesium sulfate and concentrated under reduced pressure to obtain 8.62 g of 3-(2,3,4,5,6-pentafluorophenyl)-1,4,2- Dioxazol-5-one, yield 32.77%, purity 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com