Method for separating and determining rucotinib phosphate and impurities by high performance liquid chromatography
A technology of high performance liquid chromatography and alu phosphate, which is applied in the field of high performance liquid chromatography for separation and determination of ruxolitinib phosphate and impurities, and can solve the problem of inapplicability and influence on the content of active ingredients of the main drug, the production efficiency of pharmaceutical companies and the qualified rate of finished products. Adverse effects and other problems, to achieve the effect of strong specificity, good specificity and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
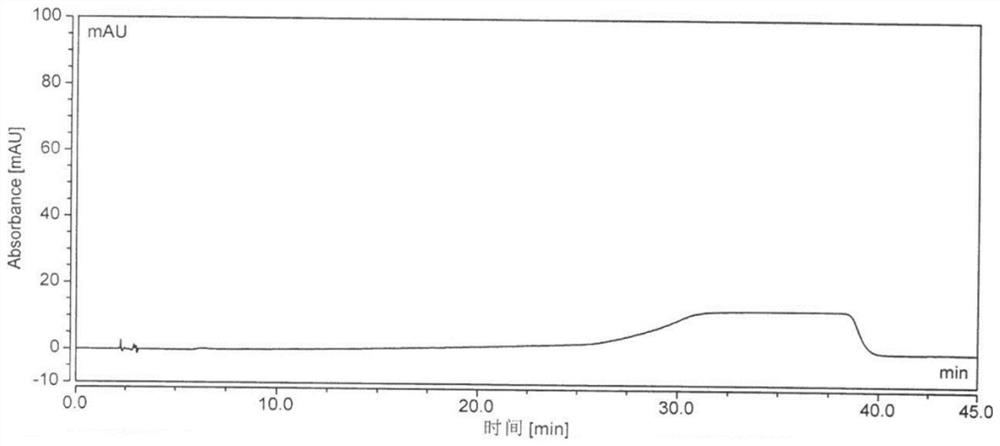
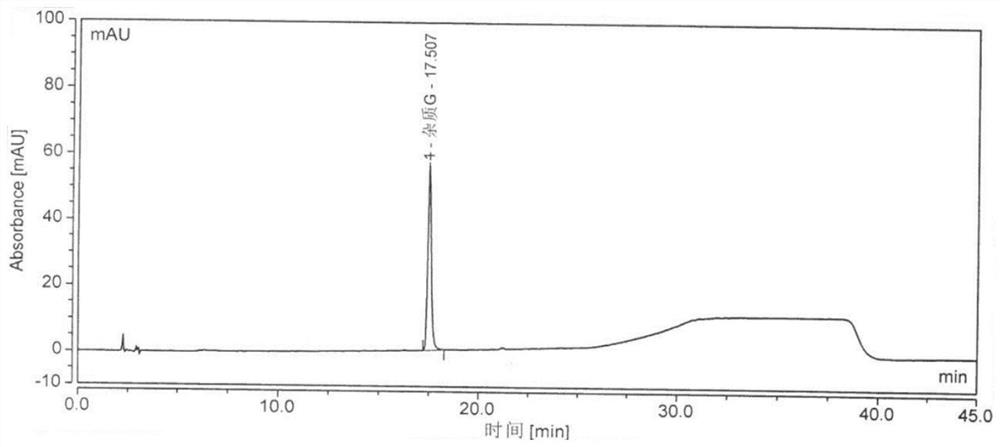
Image
Examples
Embodiment 1
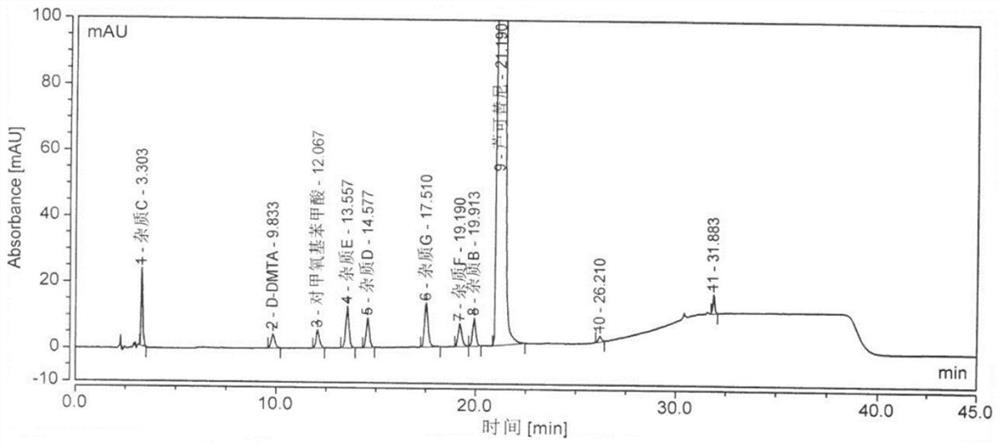
[0079] This embodiment specifically records the method for separating ruxolitinib phosphate and impurities by high performance liquid chromatography. The specific technical scheme is: mobile phase A is potassium dihydrogen phosphate, mobile phase B is methanol-acetonitrile; Silane-bonded silica gel;
[0080] Impurities include:
[0081] The method also separates the following impurities:
[0082]
[0083] Specifically, the elution ratio is:
[0084] 0min, the volume ratio of mobile phase A to mobile phase B is 70:30,
[0085] 2min, the volume ratio of mobile phase A to mobile phase B is 70:30,
[0086] 22min, the volume ratio of mobile phase A to mobile phase B is 48:52,
[0087] 27min, the volume ratio of mobile phase A to mobile phase B is 20:80,
[0088] 35min, the volume ratio of mobile phase A to mobile phase B is 20:80,
[0089] 36min, the volume ratio of mobile phase A to mobile phase B is 70:30,
[0090] 45min, the volume ratio of mobile phase A and mobile ...
Embodiment 2
[0100] On the basis of Example 1, this embodiment further illustrates the method for the determination of ruxolitinib phosphate and impurity content by high performance liquid chromatography, that is, the relevant condition parameters are specifically defined, as follows:
[0101] 1. Elution condition parameters
[0102] In this example, high performance liquid chromatography was used to separate and measure ruxolitinib phosphate and the content of impurities. The specific experimental conditions and parameters are shown in Table 2.
[0103] Table 2 High performance liquid chromatography separation and determination of ruxolitinib phosphate and impurity condition parameters
[0104]
[0105] 2. Solution preparation
[0106] Impurity B stock solution: Accurately weigh 25.56mg of the impurity B reference substance, put it in a 25ml measuring bottle, add acetonitrile to dissolve and dilute to the mark, shake well, and you get it.
[0107] Impurity C stock solution: Accuratel...
Embodiment 3
[0124] This example records other feasible chromatographic conditions, including elution gradient ratio, buffer salt type and concentration, flow rate, column temperature, etc.
[0125] 1. Research on different elution gradient ratios
[0126] Chromatographic column: Agilent ZORBAX SB-C18 4.6mm×250mm, 5μm
[0127] Column temperature: 25°C
[0128] Flow rate: 1.0ml / min
[0129] Mobile phase A: 0.03mol / L potassium dihydrogen phosphate aqueous solution (adjust pH to 3.5 with phosphoric acid)
[0130] Mobile Phase B: Methanol
[0131] Mobile Phase C: Acetonitrile
[0132] Elution gradient ratio 1: (HPLC picture as Figure 12 , the separation degree of each impurity meets the requirements)
[0133]
[0134]
[0135] Elution gradient ratio 2: (HPLC picture as Figure 13 , the separation degree of each impurity meets the requirements)
[0136] time (min) Mobile phase A(%) Mobile phase B(%) Mobile phase C(%) 0 70 15 15 2 70 15 15 20 60 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com