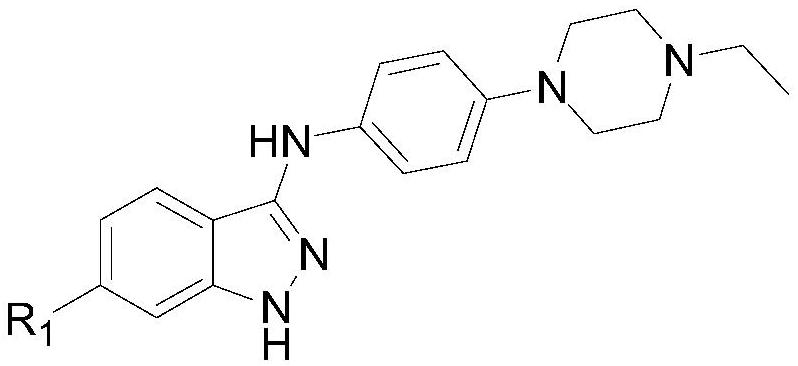
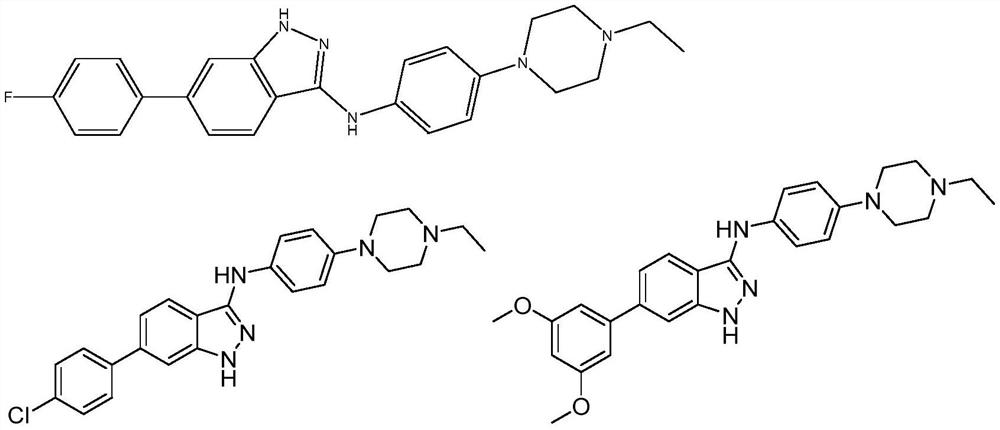
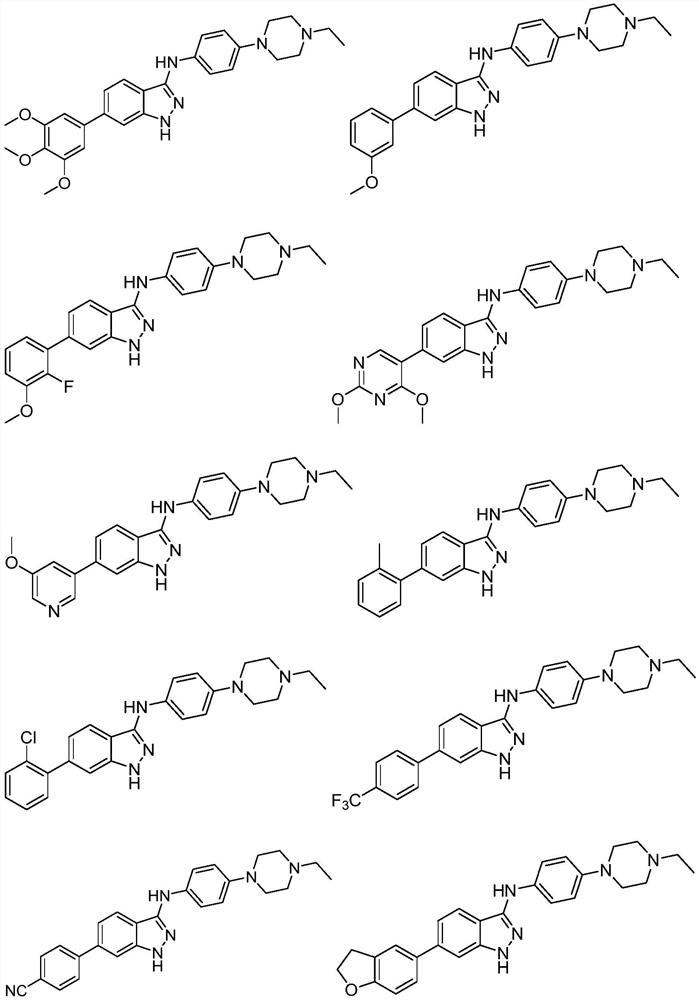
N-phenyl substituted 1H-indazole-3-amine compound and preparation and application of N-phenyl substituted 1H-indazole-3-amine compound in antitumor activity
An amine compound and antitumor drug technology, applied in the field of medicinal chemistry, can solve the problems of not widely clinical application, increase the nucleus structure of non-small cell lung cancer, and reduce the nucleus structure of non-small cell lung cancer, and achieve good inhibitory effect, Good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthesis of embodiment 1 compound
[0032] 1.1 The specific synthetic route of compound (alkali listed in route, solvent and acid-binding agent are only exemplary, not limitation of the present invention) as follows:
[0033]
[0034] The general synthetic method of reaction formula 1 compound DF1-DF16
[0035] Reagents and reaction conditions: (a) iodine, KOtBu, THF, 0℃, 2h; (b) (Boc) 2 O, DMAP, THF, rt, 2h; (c) Pd 2 (dba) 3 ,Xantphos,Cs 2 CO 3 ,N 2 , toluene, 6h.(d)Pd(pph 3 )4 ,NaOH,dioxane:H 2 O(4:1),N 2 , 90°C, react overnight.
[0036] 1.2 Examples of synthetic steps
[0037] (1) Synthesis of Intermediate 1: Step 1: Take a clean and dry 50mL round-bottomed flask and put it into a stirring bar. Dissolve 2g (10.15mmol) of 6-bromoindazole in 20mL of anhydrous THF, add 2.28g (20.30mmol) of KOtBu several times in small amounts under ice bath, and add 3.9g (15.37mmol) of iodine after 10 minutes of activation, The reaction was continued under an ice ba...
Embodiment 2
[0096] Embodiment 2 compound antitumor cell activity (kinase experiment)
[0097] 2.1 Experimental operation steps
[0098] (1) Prepare 1×Kinase buffer.
[0099] (2) Preparation of compound concentration gradients: the test compound concentration was 10 μM, repeated wells were detected, and a solution with a final concentration of 100 times was prepared in a 384-well plate. Then use Echo550 to transfer 250nl to 384 reaction plate for later use. Add 250 nl of 100% DMSO to negative control wells and positive control wells respectively.
[0100] (3) Prepare a kinase solution with a final concentration of 2.5 times with 1×Kinase buffer.
[0101] (4) Add 10 μL of 2.5-fold final concentration of kinase solution to compound wells and positive control wells; add 10 μL of 1×Kinase buffer to negative control wells.
[0102] (5) Centrifuge at 1000 rpm for 30 seconds, shake and mix well, and incubate at room temperature for 10 minutes.
[0103] (6) Use 1×Kinase buffer to prepare a mixe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com