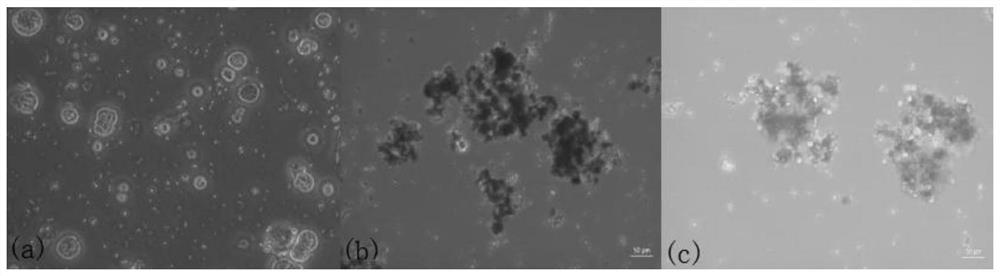
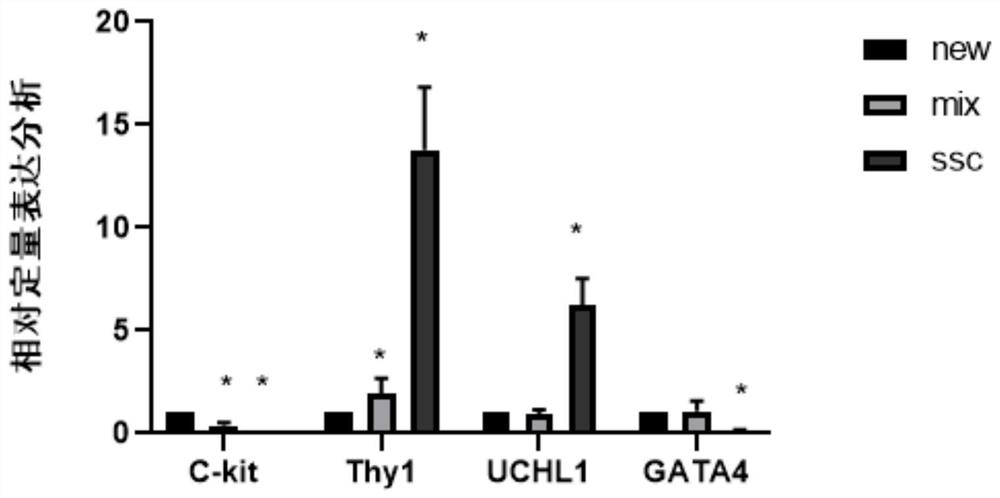
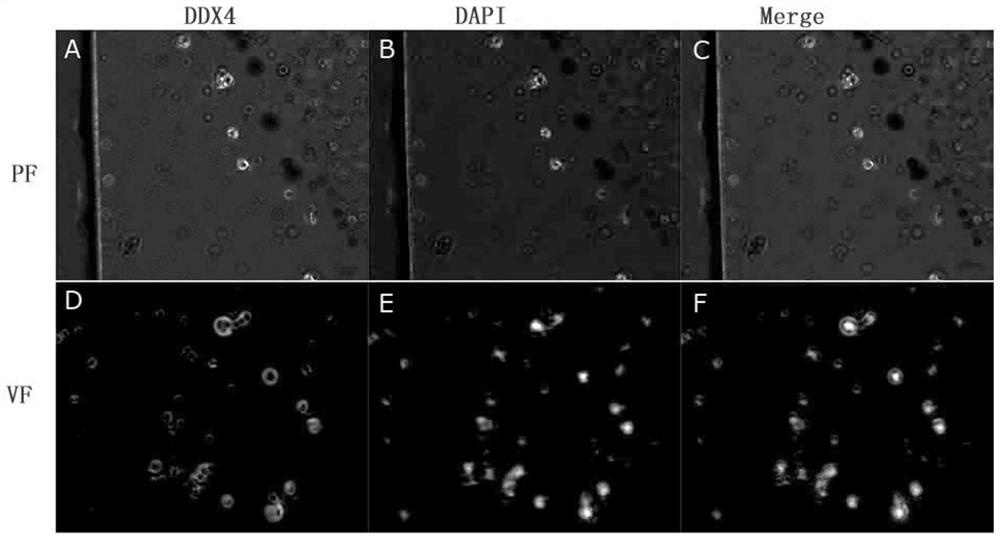
In-vitro culture method for cryopreserved bovine spermatogenic cells
A spermatogenic cell and in vitro culture technology, applied in the field of cryopreserved bovine spermatogenic cell in vitro culture, can solve problems such as inability to guarantee transportation, inability to cell culture, inability to guarantee spermatogenic cells, etc. Simple operation, the effect of reducing the number of samples and costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Fresh bovine testicular tissue was collected in sterile 0.9% NaCl solution and transported to the laboratory on melting ice;
[0059] Place three sterile Petri dishes with a diameter of 100 mm on ice and fill them with 5 mL of DMEM / F12, and use sterile forceps to transfer fresh testicular tissue into the first Petri dish filled with DMEM / F12;
[0060] Transfer fresh bovine testis tissue to a second Petri dish containing DMEM / F12;
[0061] Use sterile scissors or a scalpel to divide fresh bovine testicular tissue into 4cm 3 Remove the residual buffy coat, and keep a piece of fresh bovine testicular tissue as a fresh control;
[0062] Transfer the cut fresh bovine testicular tissue fragments to the last Petri dish containing DMEM / F12;
[0063] Place the cryovials at 4°C, fill 15 cryovials with 800 μL of cryoprotectant, fill each cryovial with four cut pieces of fresh bovine testicular tissue, and mark the cryovials;
[0064] Incubate cryovials at 4°C for 15 minutes;
...
Embodiment 2
[0082] Fresh bovine testicular tissue was collected in sterile 0.9% NaCl solution and transported to the laboratory on melting ice;
[0083] Place three sterile Petri dishes with a diameter of 100 mm on ice and fill them with 5 mL of DMEM / F12, and use sterile forceps to transfer fresh bovine testicular tissue to the first Petri dish filled with DMEM / F12;
[0084] Transfer fresh bovine testis tissue to a second Petri dish containing DMEM / F12;
[0085] Divide fresh bovine testicular tissue into 4 cm sections with sterile scissors or a scalpel 3 Remove the residual buffy coat, and keep a piece of fresh bovine testicular tissue as a fresh control;
[0086] Transfer the cut fresh bovine testicular tissue fragments to the last Petri dish containing DMEM / F12;
[0087] Place the cryovials at 4°C, fill 15 cryovials with 800 μL, 50% cryoprotectant respectively, fill each cryovial with four cut pieces of fresh bovine testicular tissue, and place them at room temperature for 10 minutes;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com