New application of MAT2A inhibitor for treating asthma
A technology for inhibitors and asthma, applied in the field of medicine, can solve problems that have not been reported yet, and achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] All experimental mice were of C57BL / 6 background and matched for sex and age. All experimental mice were raised in SPF-grade rooms in the Experimental Animal Center of Tsinghua University, with a temperature of 22-26°C and a circadian rhythm of 12 / 12 hours. All operations were strictly complied with the national and Tsinghua University laboratory animal welfare rules and regulations, and the relevant regulations formulated by Tsinghua University Laboratory Animal Use and Management Committee (IACUC).
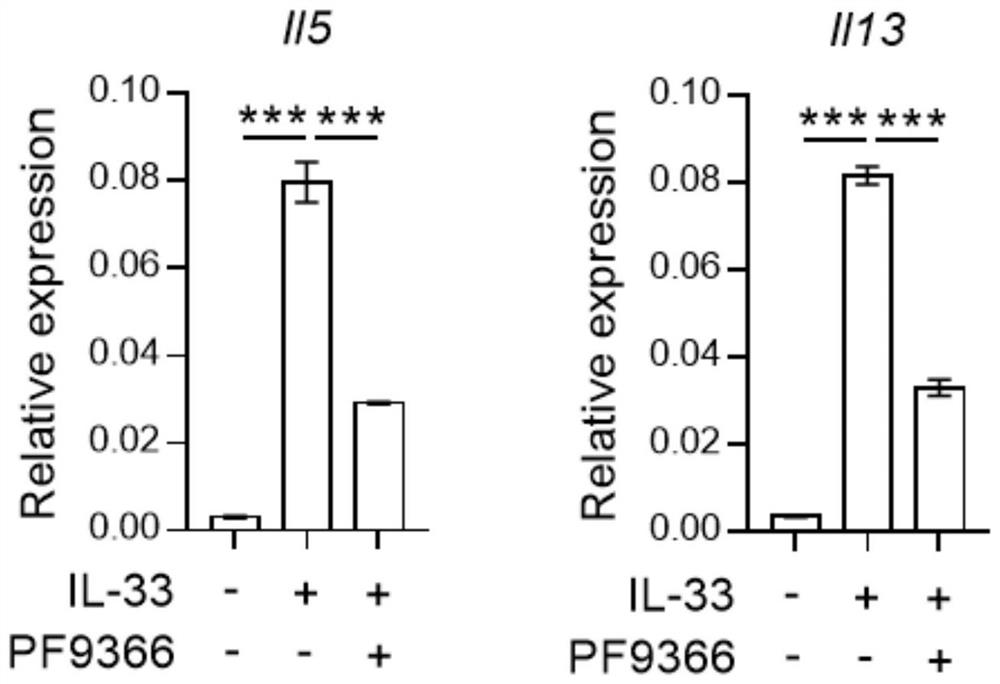
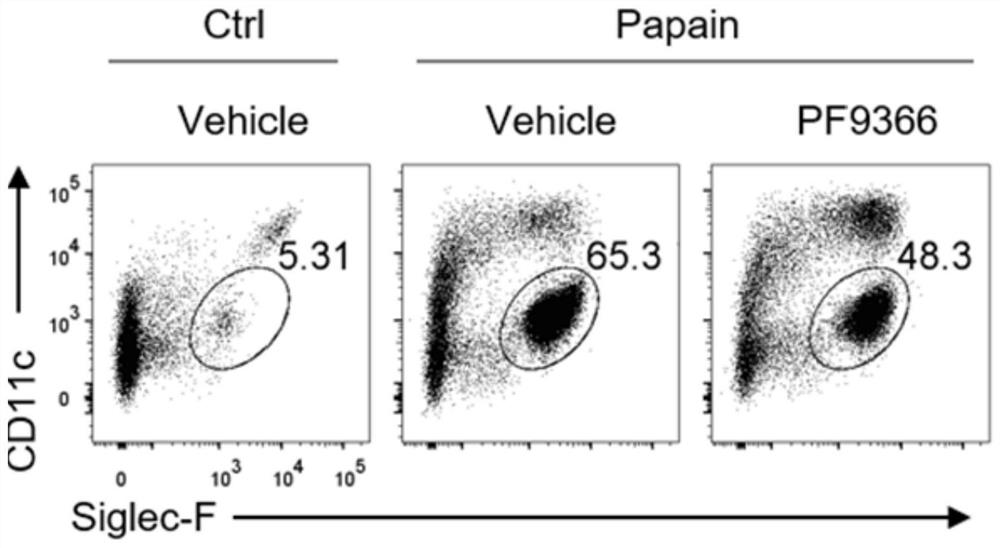
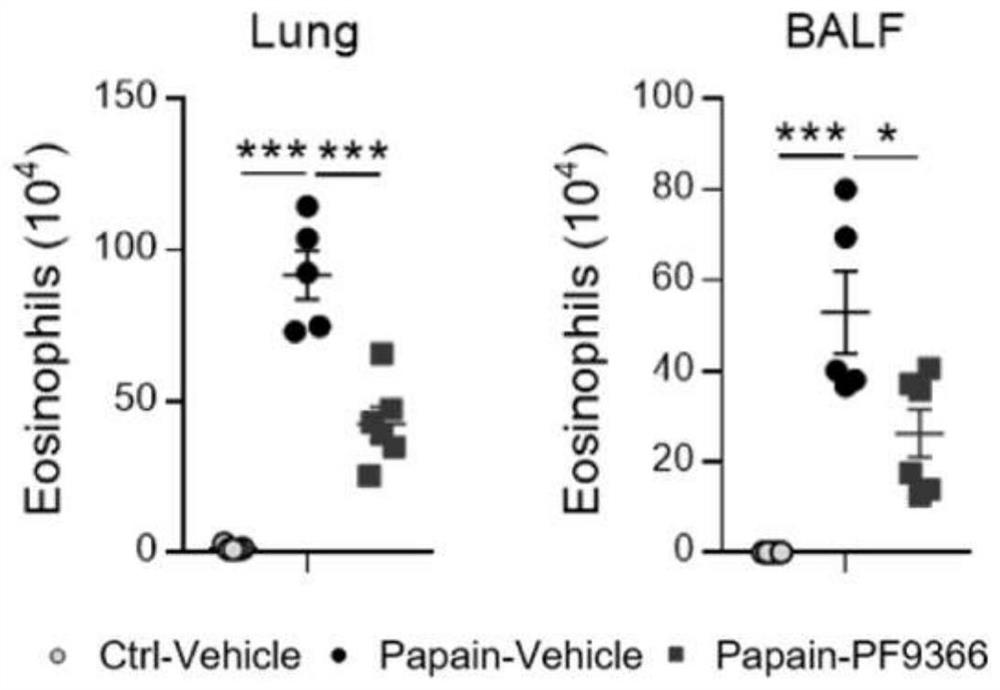
[0034] To establish an acute asthma model, mice were anesthetized with isoflurane gas, and then 20 μg of papain (both dissolved in 40 μl of 1×PBS) was given by nasal instillation. Mice were intranasally treated with papain on days 0, 1, and 3, and analyzed on day 4. For PF9366 administration, 40 mM PF9366 stock solution was first prepared with DMSO, then diluted with 1×PBS, and 15 μg PF9366 (volume 40 μl) was administered to mice by nasal drops on days -1 to 3.
[0035]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com