Triphenylamine derivative with mechanochromic characteristic and preparation method and application thereof
A technology of triphenylamine and its derivatives, which is applied in the field of triphenylamine derivatives and its preparation, to achieve the effect of good circulation and high contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
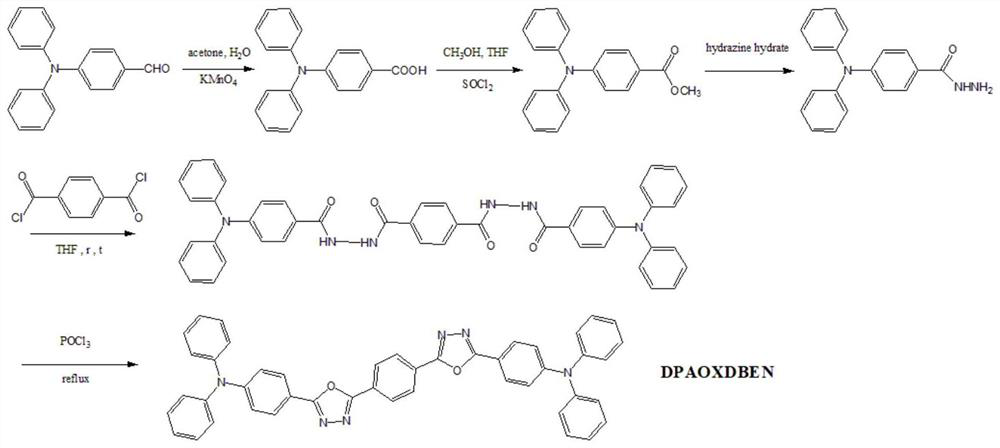
[0035] A kind of triphenylamine derivative with mechanochromic property, the name of this triphenylamine derivative is 1,4-bis{[4-4-diphenylamino]1,3,4-oxadiazole}benzene (denoted as DPAOXDBEN), has the structure shown in formula (I):
[0036]
[0037] The preparation method of above-mentioned triphenylamine derivative, comprises the steps:
[0038] 1) Synthesis of 4-dianilinobenzoic acid
[0039] Weigh 5g of 4-dianilinobenzaldehyde and pour it into an Erlenmeyer flask; add 50mL of acetone and water (volume ratio=4:1) mixed solution; slowly add 7.23g of potassium permanganate powder under stirring , and then heated and stirred to reflux for 8 hours. After the reaction is complete, first cool the solution to room temperature, spin the solvent dry with a vacuum rotary evaporator; then add an appropriate amount of distilled water to it, and filter; then add a few drops of 10% hydrochloric acid to the obtained filtrate, stir, and white flocs A precipitate was formed and filt...
Embodiment 2
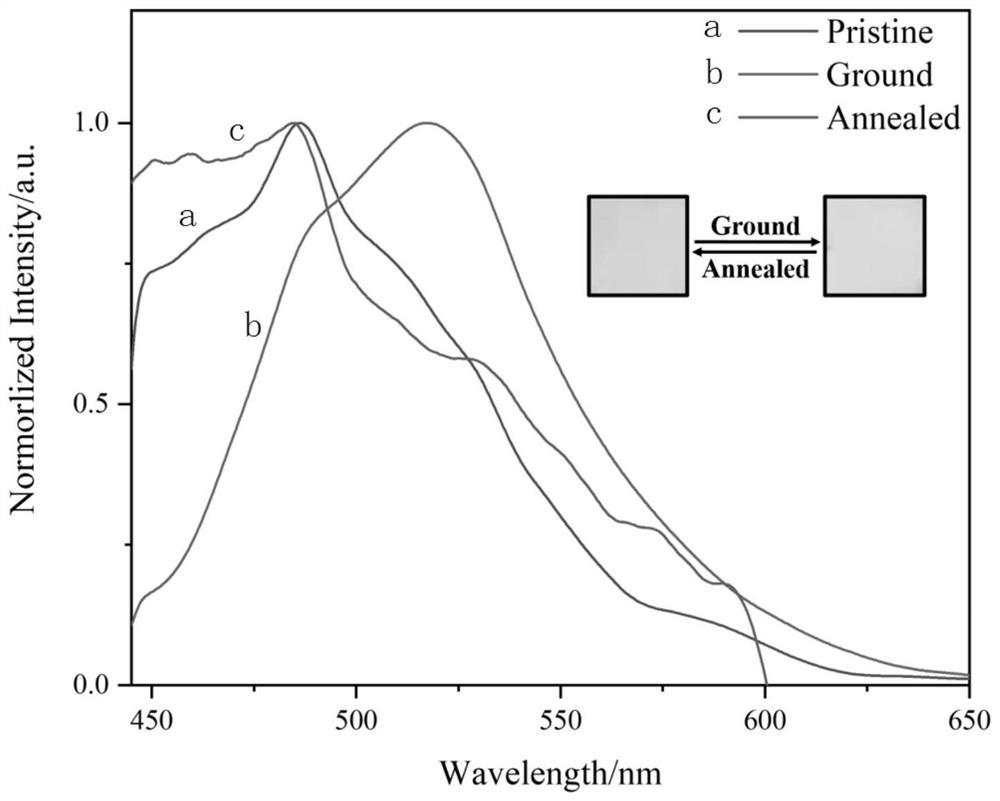
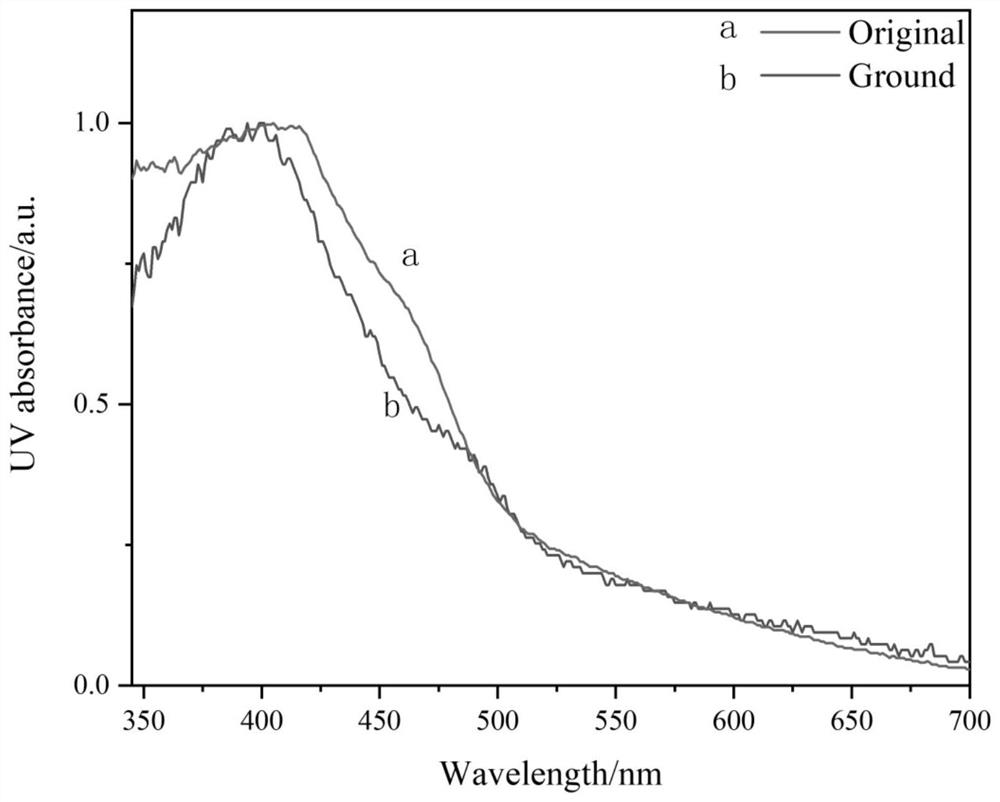
[0047] To study the molecular mechanofluorescence properties, the solid DPAOXDBEN was ground for about 20 minutes. Such as figure 2 As shown, the fluorescence spectrum of DPAOXDBEN solid has an emission peak at 486nm, and the fluorescence color is cyan. After grinding, the emission peak red shifts to 518nm, and the fluorescence color changes to green. After grinding, the powder is annealed at 150°C for 15 minutes, The fluorescence emission returns to the initial state. It can be used in smart windows, security dyes, optical records, sensors, trademark anti-counterfeiting, memory storage and display fields and other fields.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com