Application of RABGGTB in diagnosis and treatment of amyotrophic lateral sclerosis
A technology for lateral sclerosis, muscular atrophy, applied in the field of biomedicine, which can solve the problem of lack of treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1. Overexpression of RABGGTB has a protective effect on SOD1G93A motor neurons
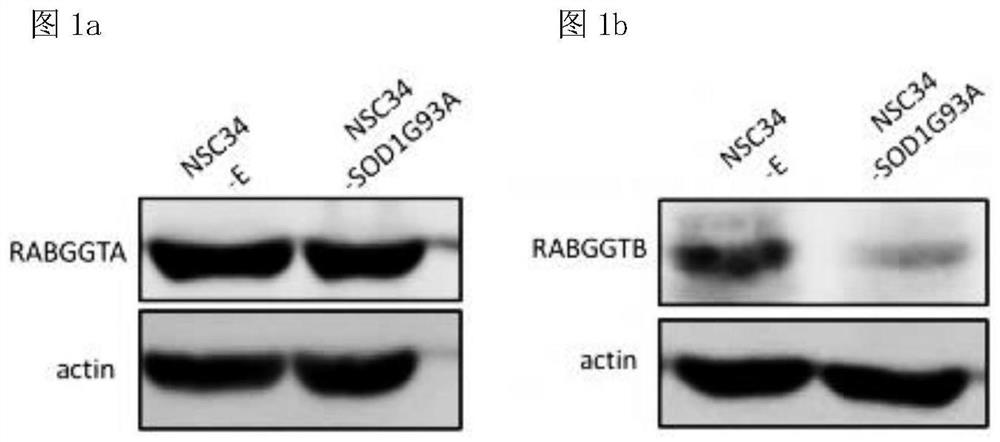
[0083] First, we verified the expression of endogenous RABGGTA and RABGGTB in NSC34-E and NSC34-hSOD1G93A cells, and the results showed that the expression of endogenous RABGGTA (α subunit of GGTase II) in NSC34-SOD1G93A cells was not significantly higher than that in NSC34-E Compared with NSC34-E, the expression of endogenous RABGGTB (β subunit of GGTase II) was significantly reduced ( figure 1 ).
[0084] Then, we overexpressed RABGGTB in ALS cell model: NSC34-hSOD1G93A cells by lentiviral transfection.
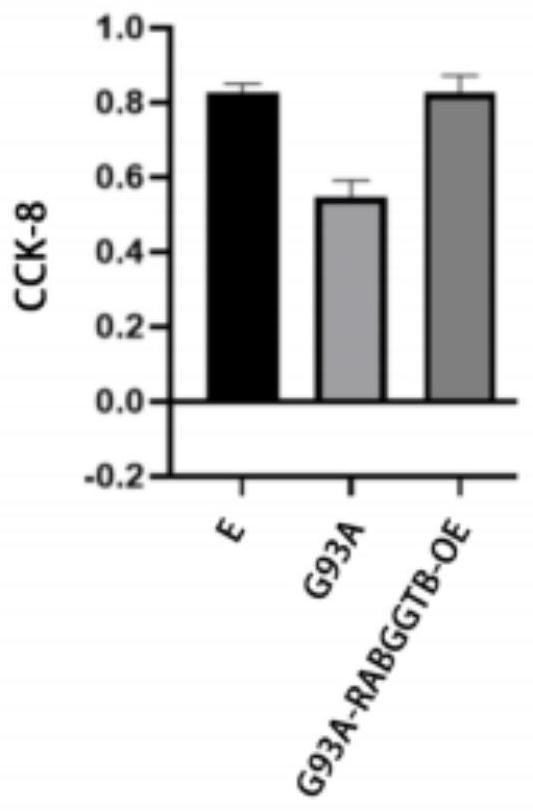
[0085] The results are shown in the figure: compared with NSC34-E, the viability of NSC34-hSOD1G93A cells decreased, and the CCK8 value of NSC34-hSOD1G93A cells increased after overexpression of RABGGTB, indicating that the cell viability was significantly increased ( figure 2 ).
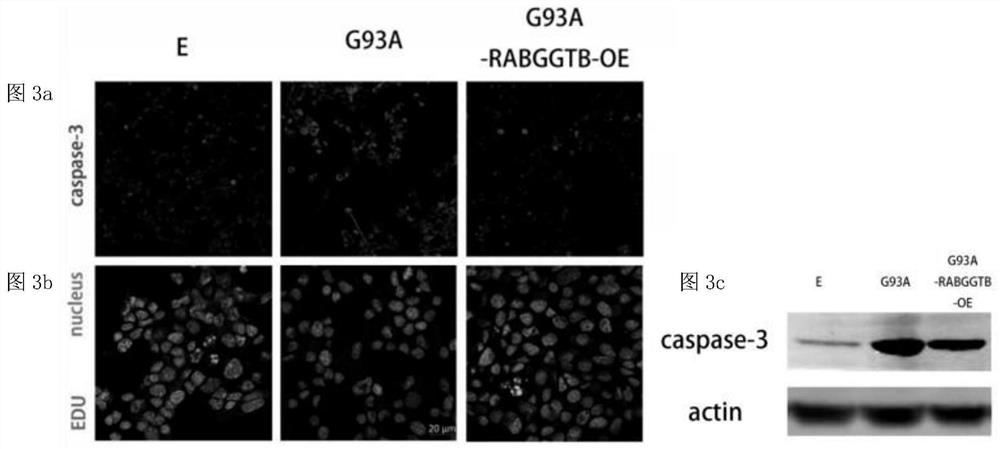
[0086] Compared with NSC34-E, cleaved caspase-3 in NSC34-hSOD1G93A cells increased, and the ex...
Embodiment 2
[0088] Example 2. Overexpression of RABGGTB reduces SOD1 protein aggregation in NSC34-SOD1G93A cells
[0089] There is a large amount of SOD1 protein aggregation in the NSC34-hSOD1G93A cell model. Previous studies have confirmed that the abnormal aggregation of SOD1 protein in motor neurons is an important cause of neuron death, which is related to the occurrence of ALS. We detected the accumulation of SOD1 in each group of cells by immunofluorescence experiments.
[0090] The results showed that compared with NSC34-E, there was a large amount of accumulated SOD1 in NSC34-hSOD1G93A motor neurons, and the level of SOD1 in NSC34-hSOD1G93A motor neurons was significantly lower after overexpression of RABGGTB ( Figure 4 ), consistent with immunofluorescence results, Western blotting results showed that overexpression of RABGGTB reduced SOD1 aggregation in NSC34-hSOD1G93A cells ( Figure 5 ).
Embodiment 3
[0091] Example 3. Overexpression of RABGGTB improves autophagy in NSC34-hSOD1G93A cells
[0092] Studies have reported that NSC34-hSOD1G93A cells have abnormal autophagy, which leads to the inability to degrade the abnormally aggregated SOD1 protein. In order to clarify whether the overexpression of RABGGRTB affects autophagy, we detected the level of autophagy in each group of cells by immunofluorescence and Western blotting. The results showed that overexpression of RABGGTB could reduce the increased levels of LC3 and P62 in NSC34-hSOD1G93A cells ( Figure 6 , Figure 7 ), and improved the impairment of autophagic flux present in NSC34-hSOD1G93A cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com