Nanometer magnetic particle chemiluminiscence detection kit for hypersensitive cardiac troponin I and preparation method and application of nanometer magnetic particle chemiluminiscence detection kit
A technology for chemiluminescence detection and cardiac troponin, which is applied in the fields of chemiluminescence/bioluminescence, analysis through chemical reactions of materials, biological testing, etc. It can solve the problems of complex biochemical properties of troponin and inaccurate detection results , to achieve fast and sensitive determination, improved sensitivity, and good system stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] In yet another embodiment of the present invention, the preparation method of this hypersensitive cTnI detection kit is provided, comprising the following steps:
[0032] 1. Preparation of M reagent
[0033] 1.1 Preparation of two cTnI-biotin antibodies
[0034] The cTnI monoclonal antibody was labeled with biotin ester, and two kinds of cTnI-biotin antibodies were prepared. The epitopes targeted by the two naked antibodies were different; among them: the antigen targeted by the first human cTnI monoclonal antibody The epitope is Troponin I-Cardiac (41-49Region); the epitope of the second anti-human cTnI monoclonal antibody is Troponin I-Cardiac (87-91Region)
[0035] 1.2 Two cTnI-biotin antibodies were coupled with streptavidin magnetic beads respectively, and the biotinylated cTnI antibody was coated on the streptavidin magnetic beads.
[0036] 1.3 The above reaction was carried out at room temperature, the coating buffer used was Tris buffer, and finally the volume...
Embodiment 1
[0049] Embodiment 1: Nano-magnetic particles chemiluminescent detection kit for ultra-sensitive cardiac troponin I
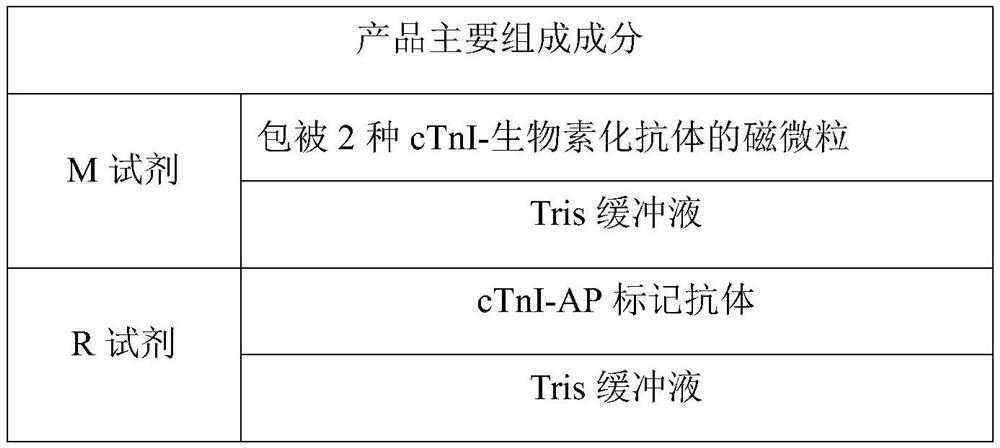
[0050] 1. Kit composition:
[0051] (1) M reagent: magnetic particles coated with the first anti-human cTnI monoclonal antibody and the second anti-human cTnI monoclonal antibody; Tris buffer.
[0052] (2) R reagent: alkaline phosphatase (AP) labeled third anti-human cTnI monoclonal antibody; Tris buffer.
[0053] 2. Preparation of the kit:
[0054] (1) The first anti-human cTnI monoclonal antibody and the second anti-human cTnI monoclonal antibody were respectively subjected to a labeling reaction and coupled with biotin to obtain biotinylated first anti-human cTnI monoclonal antibody and biotinylated Second anti-human cTnI monoclonal antibody; then biotinylated first anti-human cTnI monoclonal antibody and biotinylated second anti-human cTnI monoclonal antibody and streptavidin-modified magnetic particles were placed at room temperature React on a mixer for...
Embodiment 2
[0056] Example 2: Methodological investigation of nano-magnetic particle chemiluminescent detection kit for ultra-sensitive cardiac troponin I
[0057] 1. Minimum detection limit
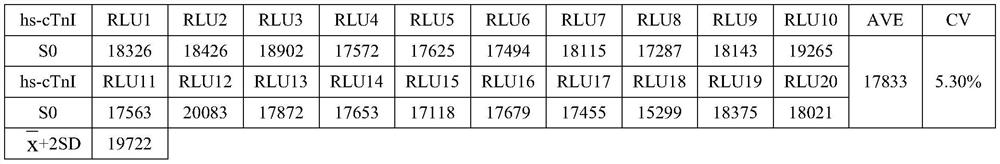
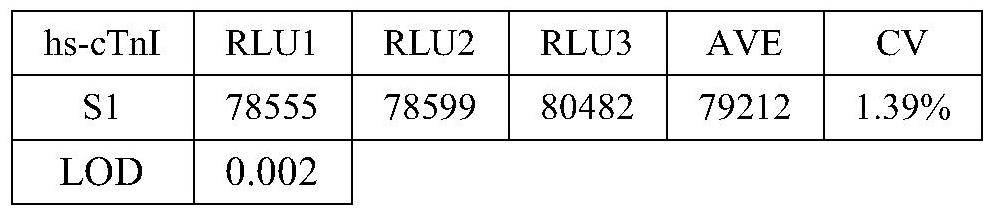
[0058] Use the zero-concentration calibrator as a sample for detection, repeat the measurement 20 times, obtain the RLU value of the 20 measurement results, and calculate the average value and standard deviation (SD). inferred According to the concentration-chemiluminescence (RLU) value results between the zero-concentration calibrator and the adjacent calibrator, a two-point regression fitting is carried out to obtain a linear equation, which is Put the corresponding RLU value into the equation to find the corresponding concentration value, the results are shown in Table 1:
[0059] Table 1: Determination results of the minimum detection limit
[0060]
[0061]
[0062] The above results show that the minimum detection limit of the nano-magnetic particle chemiluminescent detection kit ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com