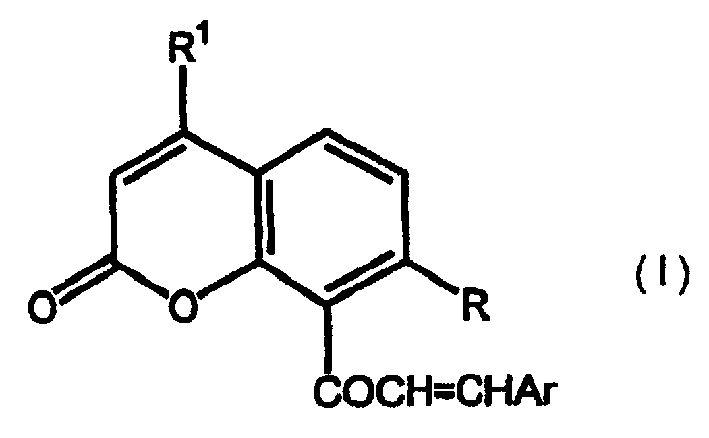
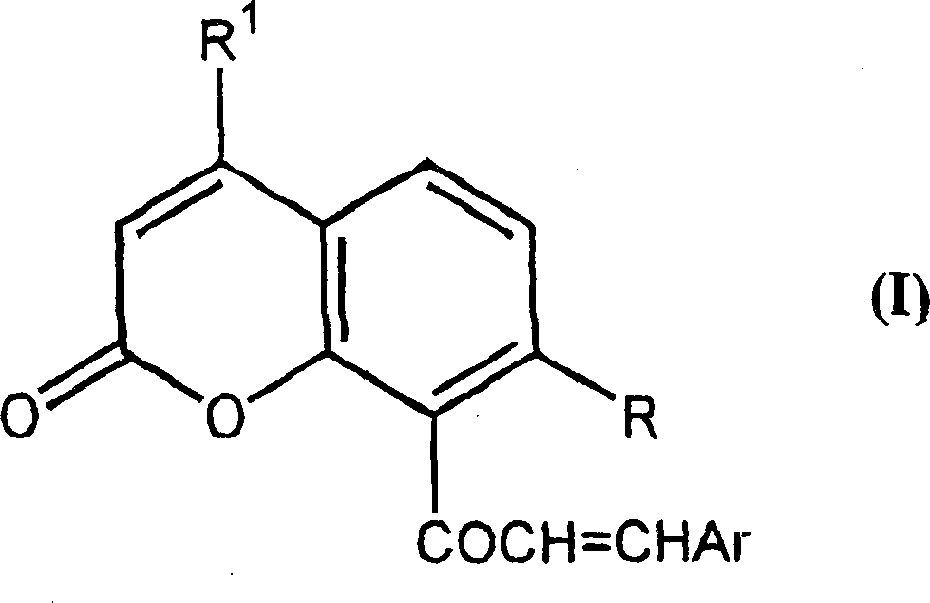
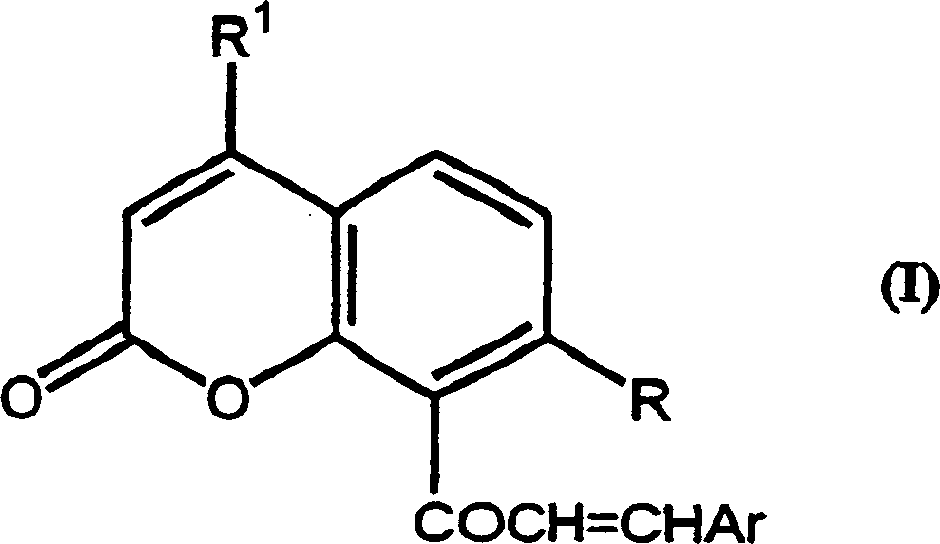
Chalcone coumarins
A technology of compounds and ring atoms, applied in the fields of organic active ingredients, sexual diseases, bone diseases, etc., can solve problems such as poor bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. General method for preparing chalcones
[0040]
[0041] Method A.
[0042] A 50% KOH solution (3 ml) was added to an equimolar solution of ketone (0.0075 mol) and aldehyde (0.0075 mol) in 95% ethanol at room temperature with vigorous stirring. The reaction was stirred overnight, then diluted with water and acidified. The precipitate was isolated by filtration and dried under vacuum. The compound was crystallized from ethanol or chromatographically followed by crystallization from ethanol. method B.
[0043] Ketone (0.0075 mol), aldehyde (0.0075 mol), piperidine (15 ml) and acetic acid (75 ml) were added countercurrently in 95% ethanol (80 ml) for 5 hours. Molecular sieves were added to the solution to remove water and left overnight. The resulting precipitate was collected and crystallized. If the product does not precipitate under these conditions, the solvent is evaporated in vacuo and the residue is purified by column chromatography on silica gel...
Embodiment 2
[0044] 50% KOH solution (3ml) was added to equimolar 4-methyl-7-(2-methylprop-1-enyloxy)-8-acetylcoumarin (2.14g, 0.0075mol) and To a solution of pyridine-3-carbaldehyde (0.8 g, 0.0075 mol) in 95% ethanol, the addition was carried out at room temperature with vigorous stirring. The reaction was stirred overnight, then diluted with water and acidified. The precipitate was isolated by filtration and dried under vacuum. Crystallization of this compound from ethanol afforded 0.84 g of product, m.p. 156-157°C, 1 H-NMR (CDCl 3 )δ: 1.69(s, 3H); 1.72(s, 3H); 2.44(d, 3H, J=1.22Hz); 4.65(d, 2H, J=6.5Hz); 5.34-5.38(m, 1H); 6.16(d, 1H, J=1.2Hz); 6.95(d, 1H, J=8.8Hz); 7.07(d, 1H, J=18Hz); 7.36(d, 1H); 7.30-7.40(m, 1H) ; 7.64 (d, 1H, J = 8.9 Hz); 7.90 (m, 1H); 8.58-8.68 (m, 2H). Example 3. 1-[4-methyl-7-(3-methylbut-2-enyloxy)coumarin-8-yl]-3-phenyl-propen-1-one (see Attached structure diagram VIB119)
Embodiment 3
[0045] A 50% solution of KOH (3ml) was added to equimolar 4-methyl-7-(3-methylbut-2-enyloxy)-8-acetylcoumarin (2.14g, 0.0075mol) and To a solution of benzaldehyde (0.8 g, 0.0075 mol) in 95% ethanol, the addition was carried out at room temperature with vigorous stirring. The reaction was stirred overnight, then diluted with water and acidified. The precipitate was isolated by filtration and dried under vacuum. Crystallization of this compound from ethanol afforded 1.34 g of product, m.p. 114-16°C, 1 H-NMR (CDCl 3 )δ: 1.69(s, 3H); 1.72(s, 3H); 2.44(d, 3H, J=1.22Hz); 4.65(d, 2H, J=6.5Hz); 5.34-5.38(m, 1H); 6.16(d, 1H, J=1.2Hz); 6.95(d, 1H, J=8.8Hz); 7.00(d, 1H, J=18Hz); 7.10(d, 1H); 7.30-7.40(m, 3H) ; 7.45-7.52 (m, 12H); 7.61 (d, 1H, J = 8.9 Hz). Example 4. 1-[4-methyl-7-(3-methylbut-2-enyloxy)coumarin-8-yl]-3-(3,4,5-trimethoxybenzene base) propen-1-one (see attached structure diagram VIB 120)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com