Method for synthesizing beta-myrcene through intermolecular nucleophilic addition reaction
An addition reaction and intermolecular technology, applied in the direction of condensation between hydrocarbons and non-hydrocarbons, organic chemistry, etc., can solve problems such as no commercial value, low energy consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment i
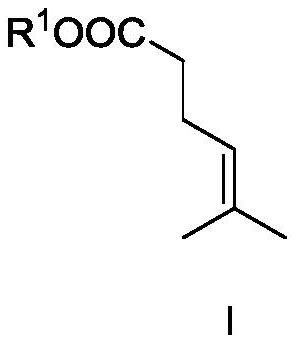
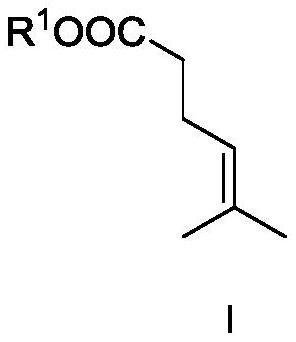
[0049] Compound I-1 was synthesized.
[0050]
[0051] In the flask, add trimethyl orthoacetate (1mol), 2-methyl-3-buten-2-ol (1mol) and propionic acid (0.2mol), then heat the reaction system to 125°C and continue to stir the reaction 12h, stop the reaction. followed by water, saturated NaHCO 3 Wash with saturated brine, dry over anhydrous sodium sulfate, and carry out vacuum distillation under the conditions of 30hPa and 70°C to obtain compound I (yield 98%). The characterization results are: 1 H NMR (400MHz, CDCl 3 ):δ1.61(s,3H),1.67(s,3H),2.30–2.35(m,4H),3.68(s,3H),5.08–5.10(m,1H); 13 CNMR (100MHz, CDCl 3 ): δ17.7, 23.4, 25.5, 34.1, 51.4, 122.3, 133.1, 173.5.
[0052] Example II
[0053] Compound 1-2 was synthesized.
[0054]
[0055] In the flask, add triethyl orthoacetate (1mol), 2-methyl-3-buten-2-ol (1mol) and propionic acid (0.2mol), then heat the reaction system to 125°C and continue to stir the reaction 12h, stop the reaction. followed by water, sat...
Embodiment 1
[0057] Synthesis of the compound β-myrcene.
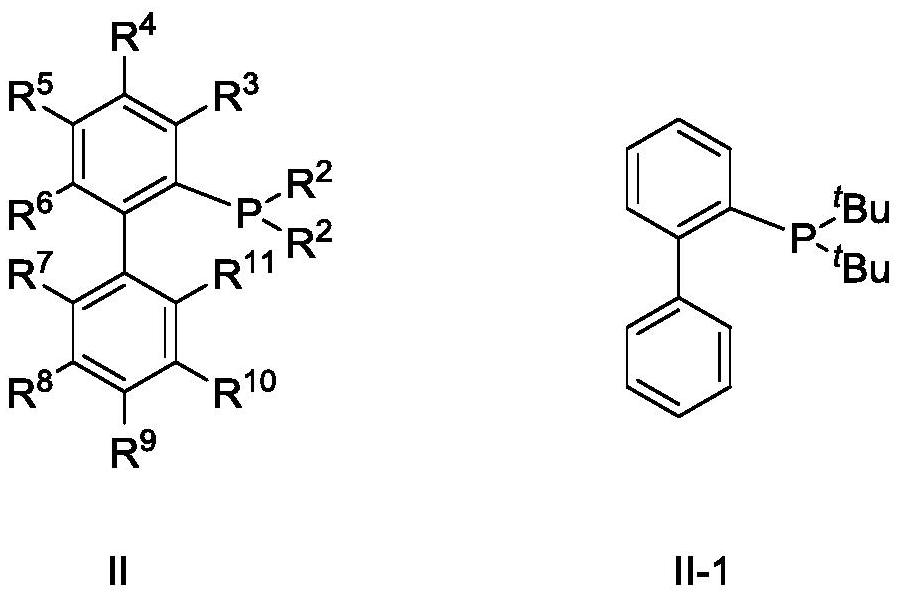
[0058] metal salt AuNTf 2 After complexing with ligand II-1, it is used as a catalyst to catalyze the reaction to prepare β-myrcene.
[0059] Under the protection of nitrogen, the metal salt AuNTf was added into the three-necked flask 2 (0.03mol, 3mol%) and ligand II-1 (0.033mol, 3.3mol%) were added to 200ml of dichloromethane, and stirred at room temperature for 1h. Then the above catalyst solution, triethylamine (2.5mol, 2.5equiv) and compound I-1 (1mol, 1equiv) in Example i were added to the pressure-resistant reactor, and vinylacetylene (1.2mol, 1.2equiv) ), and then the above system was heated to 50° C. for 12 h. Stop the reaction, cool to room temperature, discharge the remaining vinyl acetylene in the system, then raise the temperature to 115°C to continue the reaction for 6h, stop the reaction, open the reaction kettle, the reaction liquid is analyzed by the gas phase internal standard method, the conversion rate is 96%,...
Embodiment 2
[0061] Synthesis of the compound β-myrcene.
[0062] metal salt AuNTf 2 After complexing with ligand II-1, it is used as a catalyst to catalyze the reaction to prepare β-myrcene.
[0063] Under the protection of nitrogen, the metal salt AuNTf was added into the three-necked flask 2 (0.03mol, 3mol%) and ligand II-1 (0.033mol, 3.3mol%) were added to 200ml of dichloromethane, and stirred at room temperature for 1h. Then the above-mentioned catalyst solution, triethylamine (2.5mol, 2.5equiv) and compound II-2 (1mol, 1equiv) in Example i were added to the pressure-resistant reactor, and vinylacetylene (1.2mol, 1.2equiv) was passed into ), and then the above system was heated to 50° C. for 12 h. Stop the reaction, cool to room temperature, drain the remaining vinyl acetylene in the system, then raise the temperature to 115°C to continue the reaction for 6h, stop the reaction, open the reaction kettle, the reaction liquid is analyzed by the gas phase internal standard method, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com