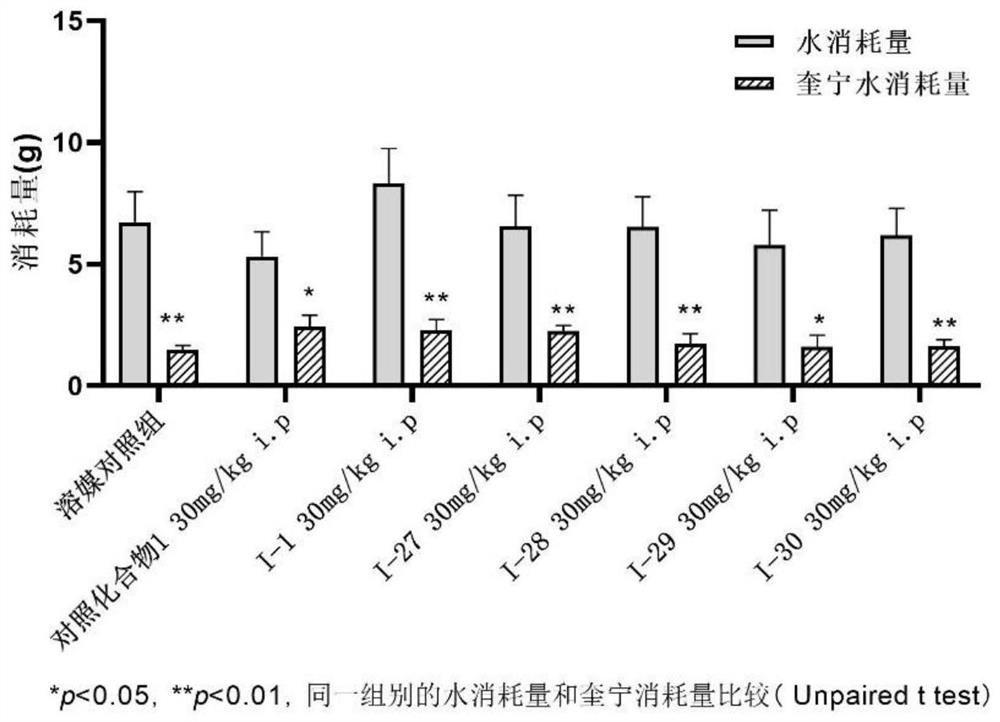
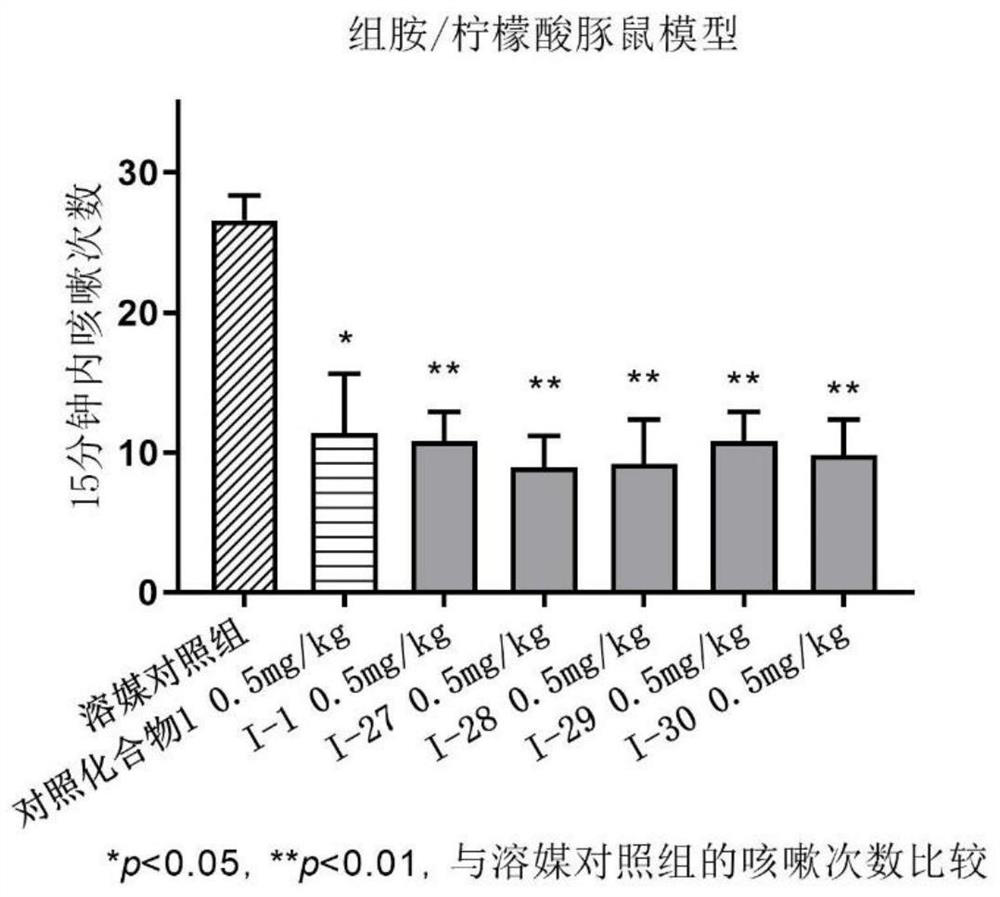
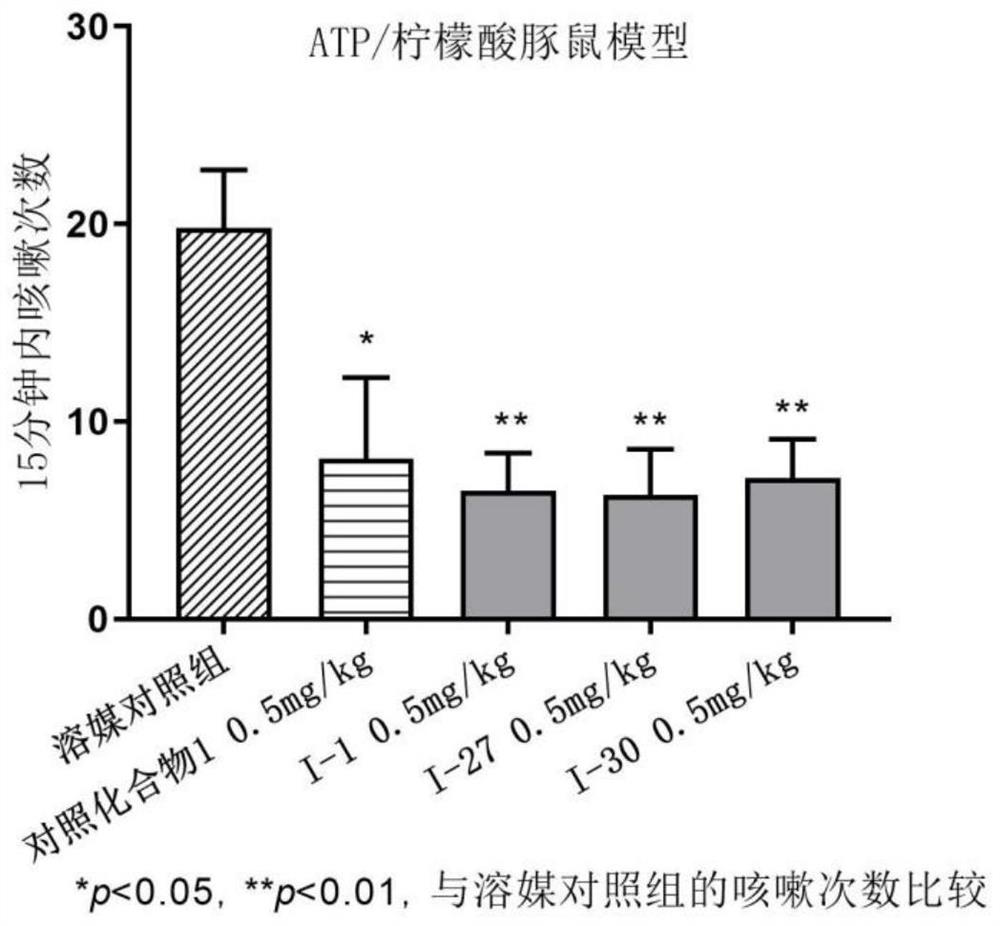
Benzamide compound and application thereof
A compound and hydrate technology, applied in the field of medicinal chemistry, can solve problems such as adverse side effects, inapplicability to patients, and unsuitability for long-term medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0274] Embodiment 1: the preparation of target compound I-1
[0275] 2-fluoro-3-(5-methylthiazol-2-yl)-5-(((R)-tetrahydrofuran-3-yl)oxy)-N-((R)-1-(2-(tri Fluoromethyl) pyrimidin-5-yl) ethyl) benzamide (target compound I-1)
[0276]
[0277] The synthetic route of target compound 1-1 is as follows:
[0278]
[0279] The first step: the synthesis of 3-bromo-2-fluoro-5-iodobenzoic acid
[0280]
[0281] 3-Bromo-2-fluorobenzoic acid (10g, 45.7mmol) was dissolved in concentrated sulfuric acid (40mL), NIS (10.27g, 45.7mmol) was added in batches at 0°C, and stirred at room temperature for three hours. Quenched with ice water (200mL), filtered, and the filter cake was washed five times with water (200mL) and dried in vacuo to obtain 3-bromo-2-fluoro-5-iodobenzoic acid as a white solid (10.9g, yield 69.2%) .
[0282] The second step: the synthesis of 3-bromo-2-fluoro-5-hydroxybenzoic acid
[0283]
[0284] Add cuprous oxide (0.656g, 4.74mmol) to a solution of 3-bromo-2...
Embodiment 2
[0308] Embodiment 2: the preparation of target compound 1-2
[0309] 2-fluoro-5-(5-methylthiazol-2-yl)-3-(((R)-tetrahydrofuran-3-yl)oxy)-N-((R)-1-(2-(tri Fluoromethyl) pyrimidin-5-yl) ethyl) benzamide (target compound I-2)
[0310]
[0311] The synthetic route of target compound 1-2 is as follows:
[0312]
[0313] The first step: the synthesis of 5-bromo-2-fluoro-3-iodobenzonitrile
[0314]
[0315] At room temperature, 2,2,6,6-tetramethylpiperidine (8.86mL, 52.5mmol) was dissolved in THF (100mL), and n-butyllithium (21.00mL, 52.5mmol, 2.5M n-hexane solution), the dropping time is more than 30min, and the reaction is stirred at -10°C for 1h, then cooled to -70°C, diethylzinc (58.0mL, 58.0mmol, 1M n-hexane solution) is added, Slowly raise the temperature to 0°C, keep the temperature and stir the reaction for 2h. Then cool to -70°C, add a solution of 5-bromo-2-fluorobenzonitrile (10g, 50.0mmol) in THF (50mL), and react at -70°C for 0.5h , and then warmed up to -30°C,...
Embodiment 3
[0322] Embodiment 3: the preparation of target compound 1-3
[0323] 4-fluoro-3-(5-methylthiazol-2-yl)-5-(((R)-tetrahydrofuran-3-yl)oxy)-N-((R)-1-(2-(tri Fluoromethyl) pyrimidin-5-yl) ethyl) benzamide (target compound I-3)
[0324]
[0325] The synthetic route of target compound 1-3 is as follows:
[0326]
[0327] The synthetic method refers to Example 1.
[0328] 1 H NMR (400MHz, CDCl 3 )δ8.91(d,2H),8.15(dd,1H),7.62-7.59(m,1H),7.55-7.46(m,1H),6.88(d,1H),5.39-5.29(m,1H) , 5.09-5.04 (m, 1H), 4.08-4.00 (m, 3H), 3.93 (td, 1H), 2.57 (d, 3H), 2.32-2.16 (m, 2H), 1.72 (d, 3H).
[0329] LC-MS, M / Z:497.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com