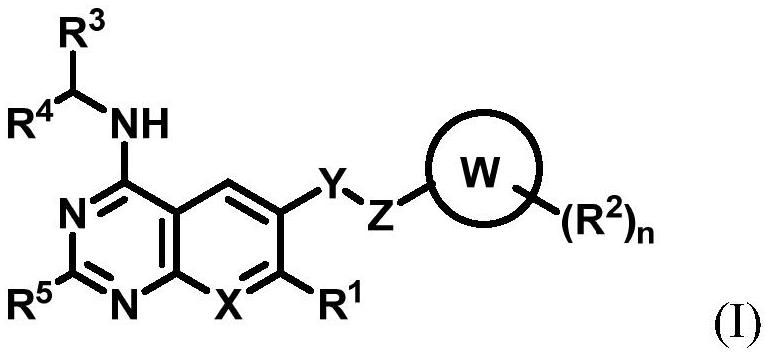
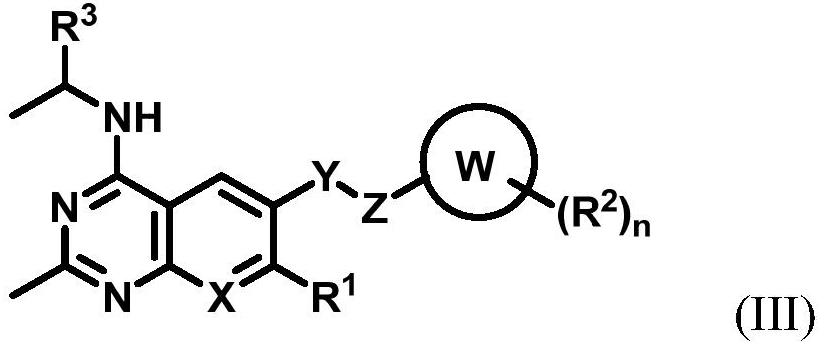
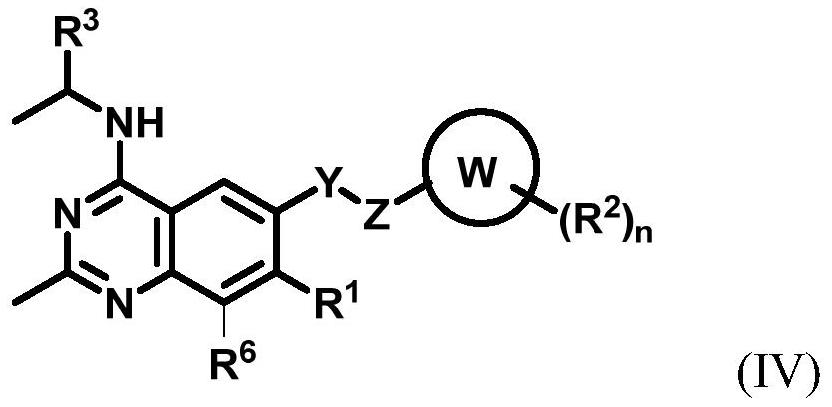
Substituted benzo or pyridopyrimidine amine inhibitor as well as preparation method and application thereof
A pyrimidine amine and benzo-based technology is applied in the field of substituted benzo- or pyrido-pyrimidine amine inhibitors and their preparation, and can solve the problems of lowering and lowering of tumor cell proliferation rate and survival rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0205] The present invention also provides a preparation method of a pharmaceutical composition, comprising the steps of: mixing a pharmaceutically acceptable carrier with the compound of general formula (I) or its crystal form, pharmaceutically acceptable salt, hydrate or The solvates are mixed to form a pharmaceutical composition.
[0206] The present invention also provides a treatment method, which includes the steps of: administering the compound of general formula (I) described in the present invention, or its crystal form, pharmaceutically acceptable salt, hydrate or solvate to the subject in need of treatment , or administer the pharmaceutical composition of the present invention for selectively inhibiting SOS1.
[0207] Compared with the prior art, the present invention has the following main advantages:
[0208] (1) The compound has a good selective inhibitory effect on SOS1;
[0209] (2) The compound has better in vivo and in vitro pharmacodynamics, pharmacokineti...
Embodiment 1
[0219] Example 1 1,1-difluoro-1-(2-fluoro-3-((R)-1-((7-methoxy-2-methyl-6-(((S)-1-methyl Preparation of ylpyrrolin-2-yl)methoxy)quinazolin-4-yl)amino)ethyl)phenyl)-2-methylpropan-2-alcohol
[0220]
[0221] The first step: the preparation of (S)-2-(chloromethyl)-1-methylpyrroline
[0222] N-methyl-L-prolinol (500.0 mg, 4.34 mmol) was dissolved in toluene (5 mL), and thionyl chloride (2.0 mL) was added. The resulting reaction solution was stirred at 100° C. for 2.0 h, and then concentrated under reduced pressure. The obtained crude product was directly submitted to the next step without further purification.
[0223] The second step: the preparation of (S)-7-methoxy-2-methyl-6-((1-methylpyrrolin-2-yl)methoxy)quinazolin-4(3H)-one
[0224] 6-Hydroxy-7-methoxy-2-methylquinazolin-4(3H)-one (120 mg, 0.58 mmol) was added to N,N-dimethylformamide (10 mL), followed by the previous step (S)-2-(Chloromethyl)-1-methylpyrroline (77.8 mg, 0.58 mmol) and potassium carbonate (402.2 mg,...
Embodiment 2
[0233] Example 2N-((R)-1-(3-amino-5-(trifluoromethyl)phenyl)ethyl)-7-methoxy-2-methyl-6-(((S)- Preparation of 1-methylpyrrolin-2-yl)methoxy)quinazolin-4-amine
[0234]
[0235] LC-MS: m / z 490 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com