Pyridine-containing triazole compound as well as preparation method and application thereof
A technology of pyridine bitriazole and compounds, which is applied in the field of inhibitor preparation, and can solve problems such as adverse reactions and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
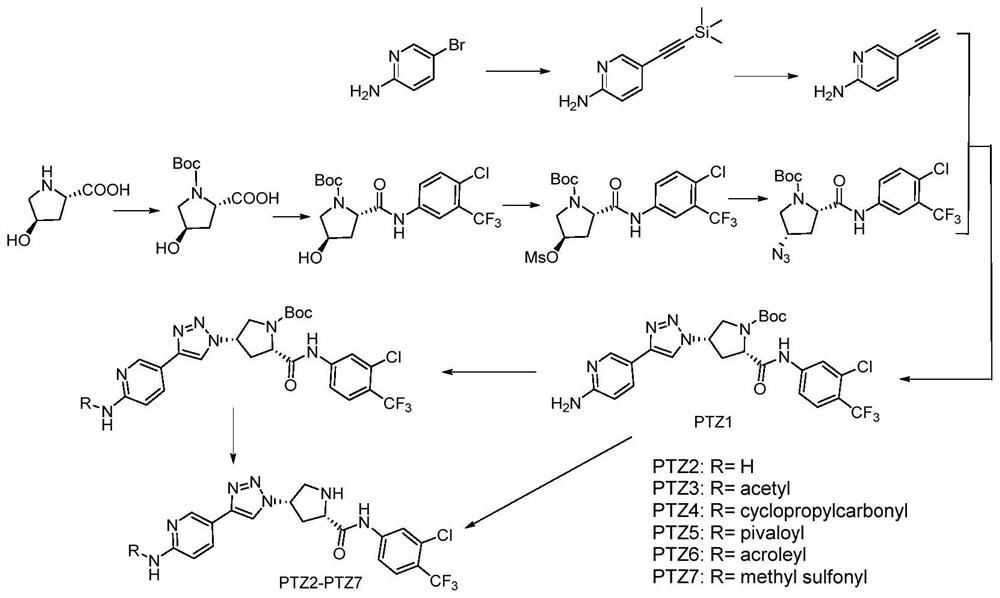
[0063] A kind of pyridine triazole compound, R is H, R' is Boc, or R is acetyl, when R' is H, the preparation method is as follows:
[0064] 1) Synthesis of 2-amino-5-(trimethylsilyl)ethynylpyridine: 2-amino-5-bromopyridine (2.79g, 16.12mmol), copper iodide (10%, 0.31g, 1.61mmol ), and tetrakis(triphenylphosphine)palladium (10%, 1.86g, 1.61mmol) were added to a 100mL double-neck round bottom flask equipped with a condenser and magnetic stirring. The vessel was then sealed with a rubber stopper, evacuated and backfilled with nitrogen 3 times. With triethylamine (30mL) as the base and solvent, inject with a syringe. After 5min at room temperature, trimethylsilylacetylene (4.74mL, 48.36mmol) was added, heated to reflux for 12h, and the reaction was complete according to TLC. The reaction was cooled to room temperature and quenched with 50 mL of water. The solution was then diluted with 50 mL of ethyl acetate and filtered. The filtrate was washed with water until the blue colo...
Embodiment 2
[0074] A kind of pyridyl triazole compound, when R is H, and R' is H, the preparation method is as follows:
[0075] Step 1) to step 7) are the same as in Example 1 to obtain (2S,4S)-4-(4-(6-aminopyridin-3-yl)-1H-1,2,3-triazole-1- yl)-2-((4-chloro-3-trifluoromethylphenyl)carbamoyl)pyrrole-1-carboxylic acid tert-butyl ester (PTZ1).
[0076] Step 8) is the same as Step 9) of Example 1, and the Boc protecting group is removed to obtain 0.25 g of compound PTZ2 with a yield of 76%. Mp 101.9~102.6℃; EI-MS (m / z) 452.10[M+H] + ,450.10[M-H] - .HRMS m / z versus C 19 h 18 CIF 3 N 7 O([M+H] + ) The calculated value is 452.12135, and the measured value is 452.12273. 1 H NMR (400MHz, DMSO-d 6 )δ10.44(s,1H),8.52(s,1H),8.33(d,J=2.3Hz,1H),8.26(d,J=2.6Hz,1H),7.97(dd,J=8.8,2.5 Hz,1H),7.73(dd,J=8.5,2.4Hz,1H),7.64(d,J=8.7Hz,1H),6.48(d,J=8.7Hz,1H),6.12(s,2H), 5.18–5.11(m,1H),4.06–4.00(m,1H),3.53–3.49(m,1H),3.36–3.31(m,1H),2.85–2.81(m,1H),2.47–2.38(m ,1H).
[0077] The synthesis steps of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com