Preparation method of plasma and serum endogenous small molecule compound quality control material
A preparation method, endogenous technology, applied in the field of testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The present embodiment provides a dialysis method. First, the collected plasma or serum is dialyzed. The dialysis bag is first boiled with 50% ethanol for 1 hour, and then successively with 50% ethanol, 0.01mol / L sodium bicarbonate and 1mmol / L EDTA ( pH=8.0) solution, and finally rinsed twice with distilled water. Add 10mL of plasma or serum to the dialysis bag, put the dialysis bag into 100ml of water at 4°C and dialyze 3 times, 1 hour each time.
[0034] After the dialysis, an appropriate amount of dialysis serum or plasma was taken to detect whether the dialysis was successful by liquid chromatography tandem mass spectrometry. The results are shown in Table 1-Table 4 respectively.
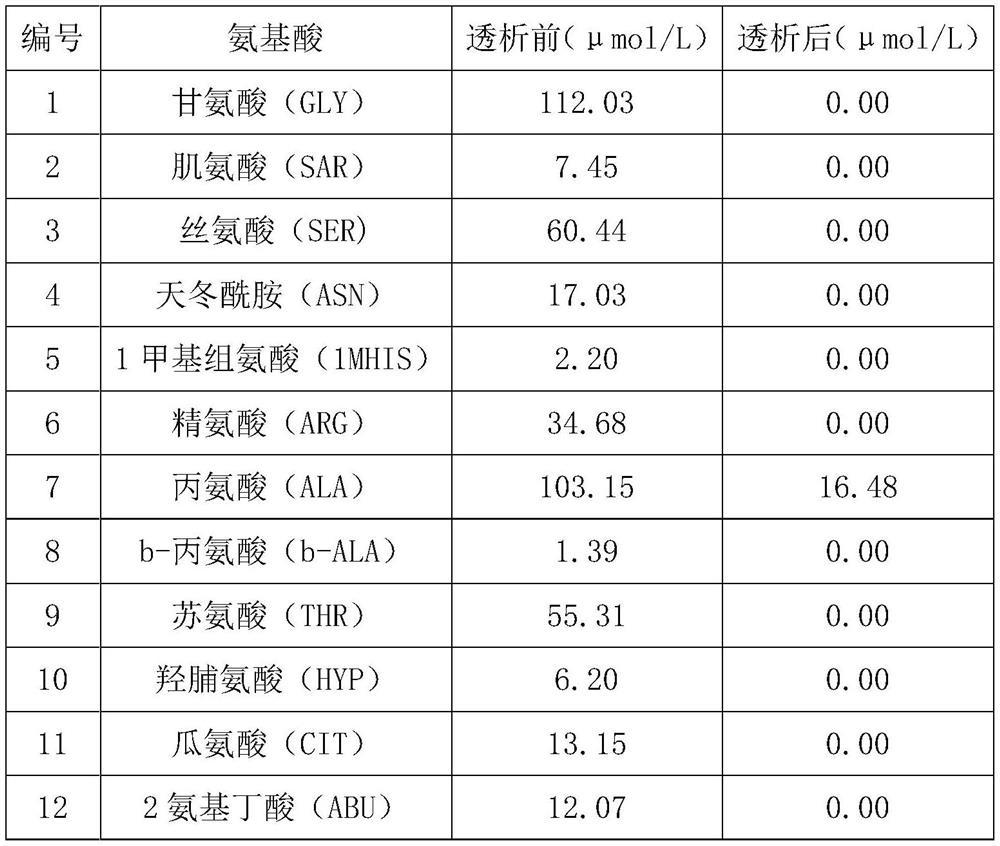
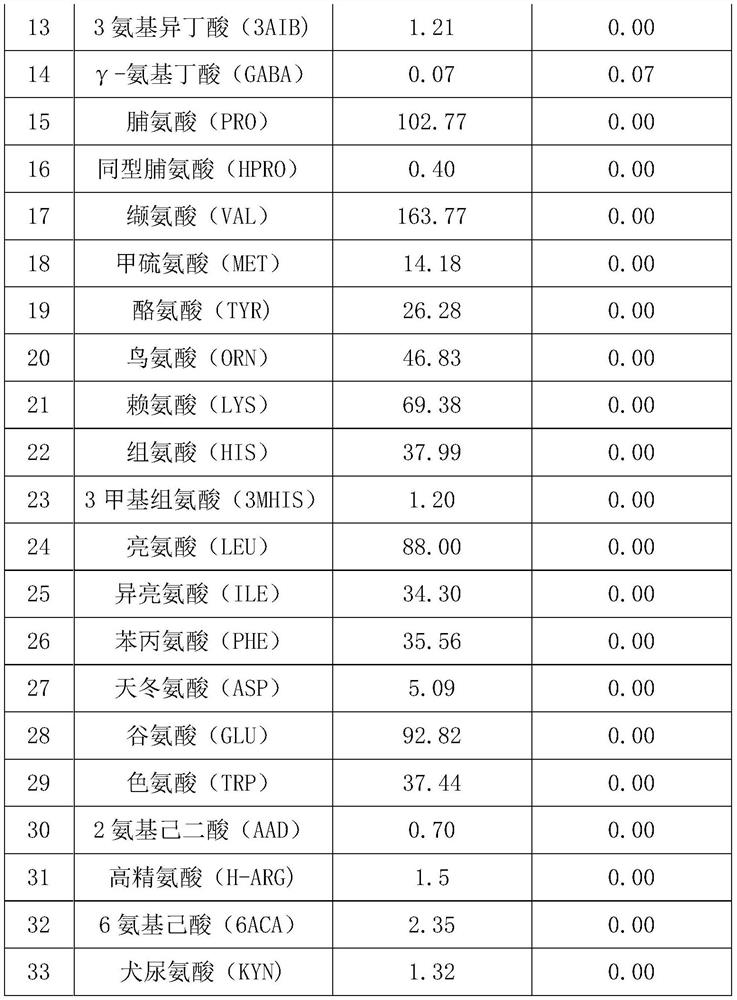
[0035] Table 1 Amino acid concentration before and after dialysis
[0036]
[0037]
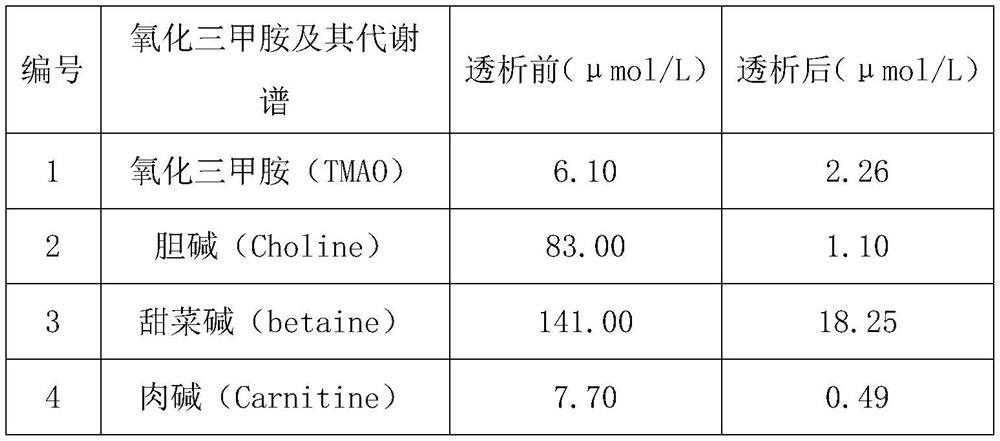
[0038] Table 2 Concentration of trimethylamine oxide and its metabolic profile before and after dialysis
[0039]
[0040]
[0041] Table 3 Concentrations of 11 steroid hormones before and ...
Embodiment 2
[0048] This embodiment provides the preparation method of amino acid low and high quality control:
[0049] Low amino acid, high quality control preparation method, take the dialyzed plasma in Example 1, add the amino acid mixture according to the clinical reference range and analysis measurement range, mix well, the ratio of plasma to amino acid mixture is 1:1-100:1, The theoretical concentration of each amino acid quality control is shown in Table 5.
[0050] Table 5 amino acid quality control concentration
[0051]
[0052]
[0053]
Embodiment 3
[0055] This example provides a preparation method for trimethylamine oxide and its metabolic profile with low and high quality control: take the dialyzed plasma in Example 1, add trimethylamine oxide and its metabolic profile mixture according to the clinical reference range and analysis measurement range, and mix well , the ratio of plasma to trimethylamine oxide and its metabolic profile mixture is 1-100:1, and the theoretical concentration of trimethylamine oxide and its metabolic profile for quality control is shown in Table 6.
[0056] Table 6 Trimethylamine oxide and its metabolic profile quality control concentration
[0057]
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com