Preparation method of Sendamaldine
A technology of azabicyclo and hexane hydrochloride, applied in organic chemical methods, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve problems such as difficult purification of crystalline polymorphs and difficulty in achieving repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
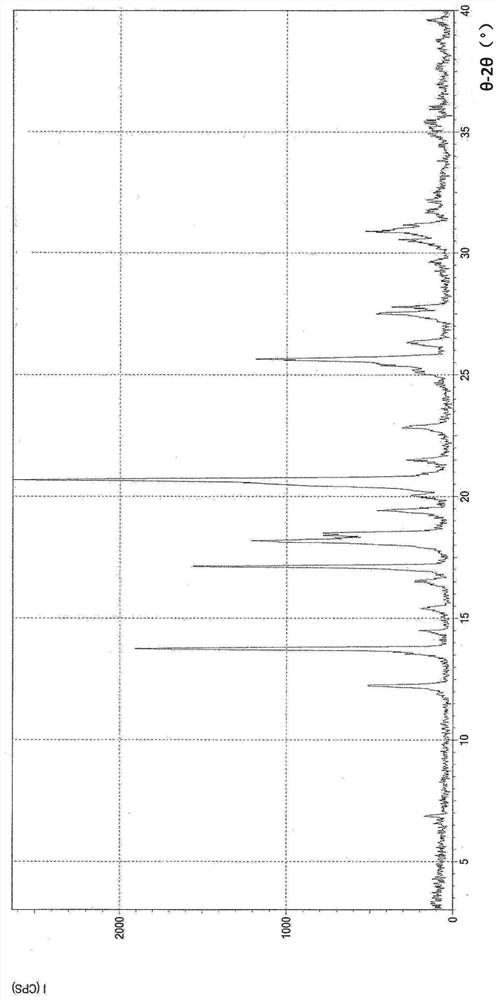
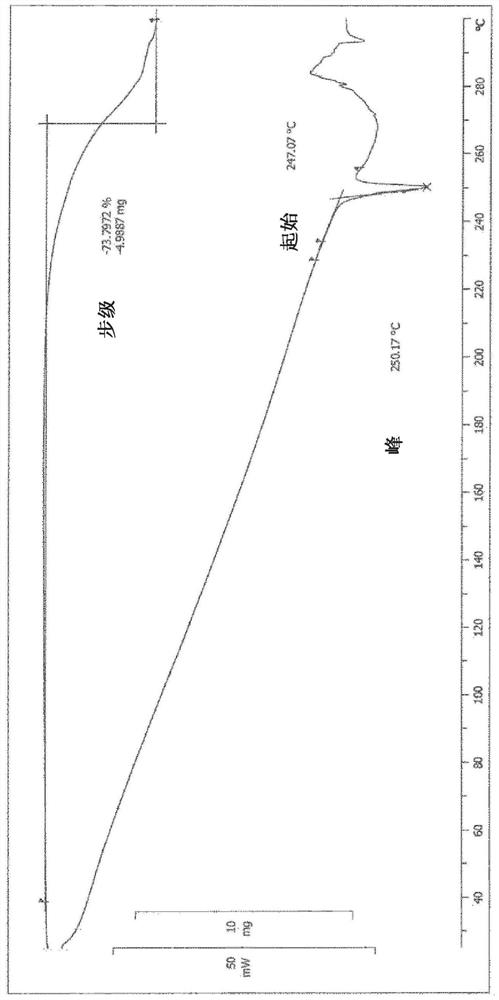
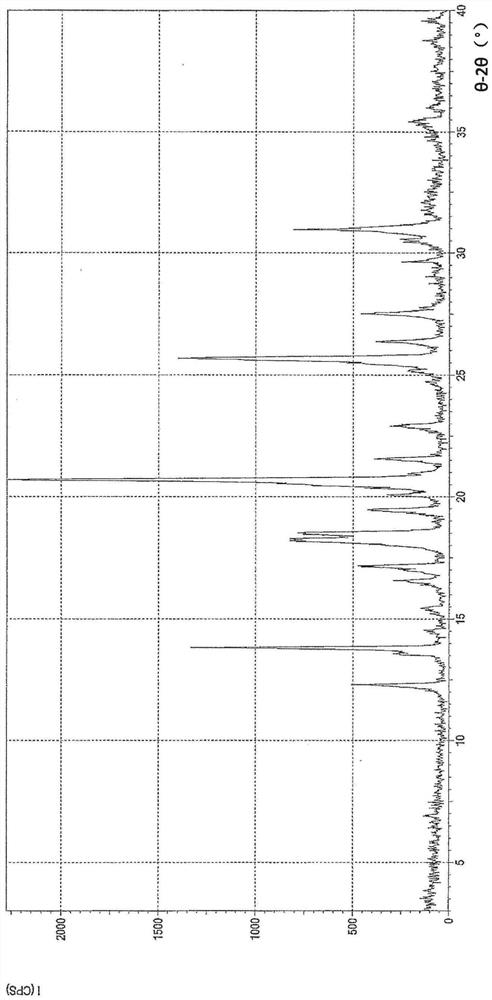
Image
Examples
Embodiment 1
[0098] Preparation of crystal form A of (1R,5S)-1-(naphthalen-2-yl)-3-azabicyclo[3.1.0]hexane hydrochloride (compound 1) (1)
[0099]
[0100] To a mixture of ethanol (178.2 mL) and water (1.8 mL) was added (1R,5S)-1-(naphthalen-2-yl)-3-azabicyclo[3.1.0]hexane hydrochloride under nitrogen atmosphere The crude product (20.0 g, 0.081 mol). The mixture was refluxed for more than 10 minutes to dissolve the crude product. The solution was left to cool until reflux ceased. To the solution was added activated carbon (2.0 g) and the mixture was refluxed for more than 30 minutes. The mixture was filtered while hot, and the activated carbon on the filter was washed with a mixture of ethanol (19.8 mL) and water (0.2 mL). The filtrate was refluxed again for more than 10 minutes (79°C) to completely dissolve the filtrate. The resulting solution was cooled to 60°C and then reheated to 70°C. The mixture was then stirred at 70°C for more than 60 minutes. The mixture was cooled to 10°...
Embodiment 2
[0105] Preparation of crystal form A of (1R,5S)-1-(naphthalen-2-yl)-3-azabicyclo[3.1.0]hexane hydrochloride (compound 1) (2)
[0106] To a mixture of ethanol (178.2 mL) and water (1.8 mL) was added (1R,5S)-1-(naphthalen-2-yl)-3-azabicyclo[3.1.0]hexane hydrochloride under nitrogen atmosphere The crude product (20.0 g, 0.081 mol). The mixture was refluxed for more than 10 minutes to dissolve the crude product. The solution was left to cool until reflux ceased. Activated carbon (2.0 g) was added to the solution and the mixture was refluxed for more than 30 minutes. The mixture was filtered while hot, and the activated carbon on the filter was washed with a mixture of ethanol (19.8 mL) and water (0.2 mL). The filtrate was refluxed again for more than 10 minutes (79°C) to completely dissolve the filtrate. The resulting solution was cooled to 51°C and then reheated to 70°C. The mixture was then stirred at 70°C for more than 60 minutes. The mixture was cooled to 10°C to precipi...
Embodiment 3
[0111] Preparation of crystal form A of (1R,5S)-1-(naphthalen-2-yl)-3-azabicyclo[3.1.0]hexane hydrochloride (compound 1) (3)
[0112] To a mixture of ethanol (178.2 mL) and water (1.8 mL) was added (1R,5S)-1-(naphthalen-2-yl)-3-azabicyclo[3.1.0]hexane hydrochloride under nitrogen atmosphere The crude product (20.0 g, 0.081 mol). The mixture was refluxed for more than 10 minutes to dissolve the crude product. The solution was left to cool until reflux ceased. Activated carbon (2.0 g) was added to the solution and the mixture was refluxed for more than 30 minutes. The mixture was filtered while hot, and the activated carbon on the filter was washed with a mixture of ethanol (19.8 mL) and water (0.2 mL). The filtrate was refluxed again for more than 10 minutes (79°C) to completely dissolve the filtrate. The resulting solution was cooled to 60°C and then reheated to 72°C. The mixture was then stirred at 72°C for over 60 minutes. The mixture was cooled to 10°C to precipitate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com