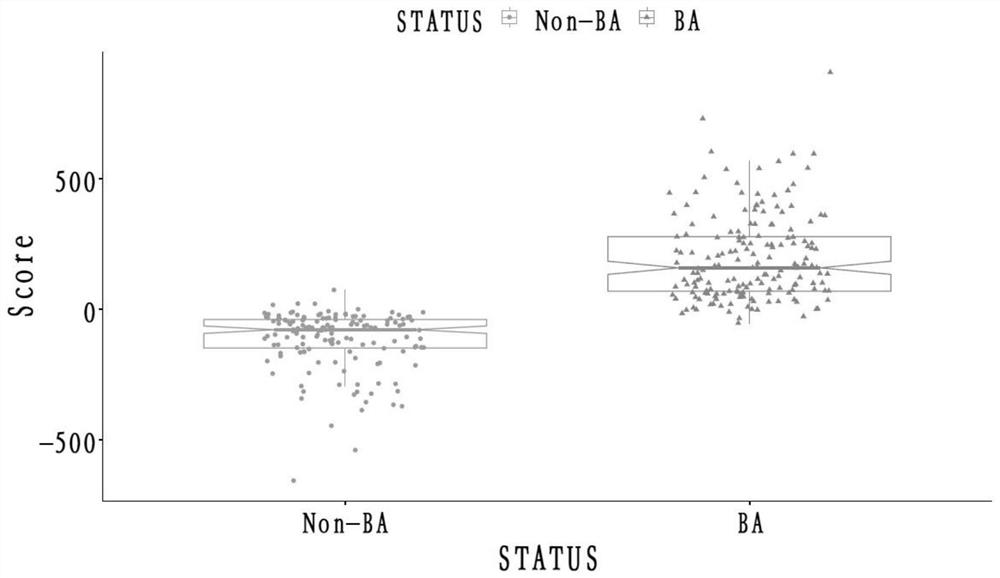
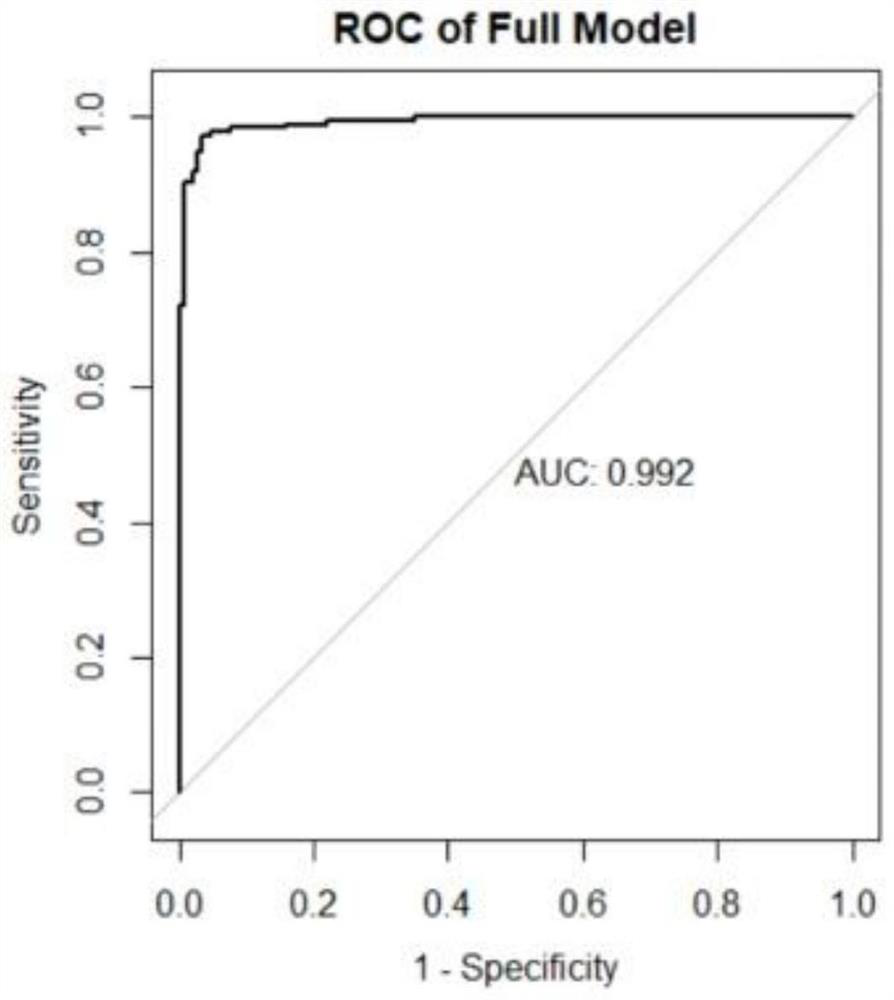
Biliary atresia evaluation system, method for detecting MMP-7, kit and application
A technology of MMP-7 and detection kits, applied in biological testing, measuring devices, material inspection products, etc., can solve the problems of false negative results, pain of children, trauma, etc., to reduce the negative rate, avoid missed diagnosis and misdiagnosis, The effect of improving the prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] This kit is limited to the detection of human serum samples.
[0085] Serum samples can be stored for 7 days under the conditions of 2°C to 8°C; and 6 months under the conditions of -15°C-25°C.
[0086] Try to avoid the repeated freezing and thawing of the sample, and the number of freezing and thawing is no more than 6 times.
[0098] 3.11 coverslips;
[0100] 4.1 Equilibrate the serum sample and the kit to room temperature (20°C to 25°C);
[0101] 4.2 Preparation of 1X washing solution: the washing solution (20X) was diluted 20 times with purified water, for subsequent use.
[0102] 4.3 Pre-diluting the serum sample: dilute the sample 20 times with the sample diluent, such as 190 μL sample dilution
[0103] 4.4 Add 100 μL of diluted samples to each well of the sample wells; add 100 μL of calibrator and 100 μL of each of the quality control products to each well of the calibration quality control wells
[0105] Automatic plate washing: wash 4 times with 1X washing solution,...
Embodiment 2
[0121] The dried blood slice quantitative collection device adopts the dried blood slice quantitative collection device disclosed by CN212513820U. Quantification of dried blood tablets
[0123] Test sample: dried blood sheet. Collection site: If blood is collected from the fingertip, take the inner side of the ring finger of the right hand; if blood is collected from the heel, take the small
[0135] Take out the 96-well plate, discard the liquid in the well, add 300 μL washing solution to each well after patting dry, and discard the washing after shaking for 30 seconds
[0148] Add 100 μL of enzyme-labeled secondary antibody, and incubate for one hour at 37°C;
[0150] Add 100 μL of substrate TMB, shake and mix, and incubate at 37° C. for ten minutes after sealing;
[0151] Add stop solution 50 μL;
Embodiment 3
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com