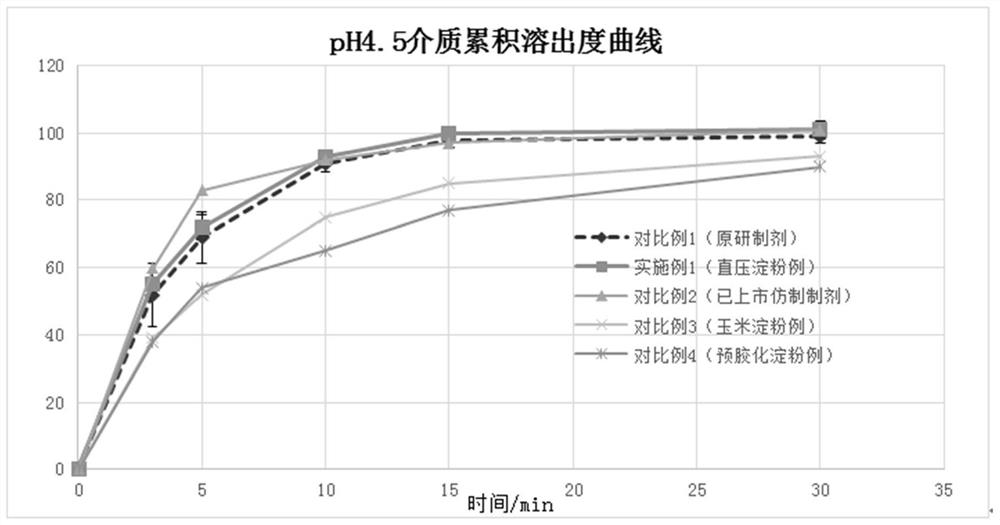
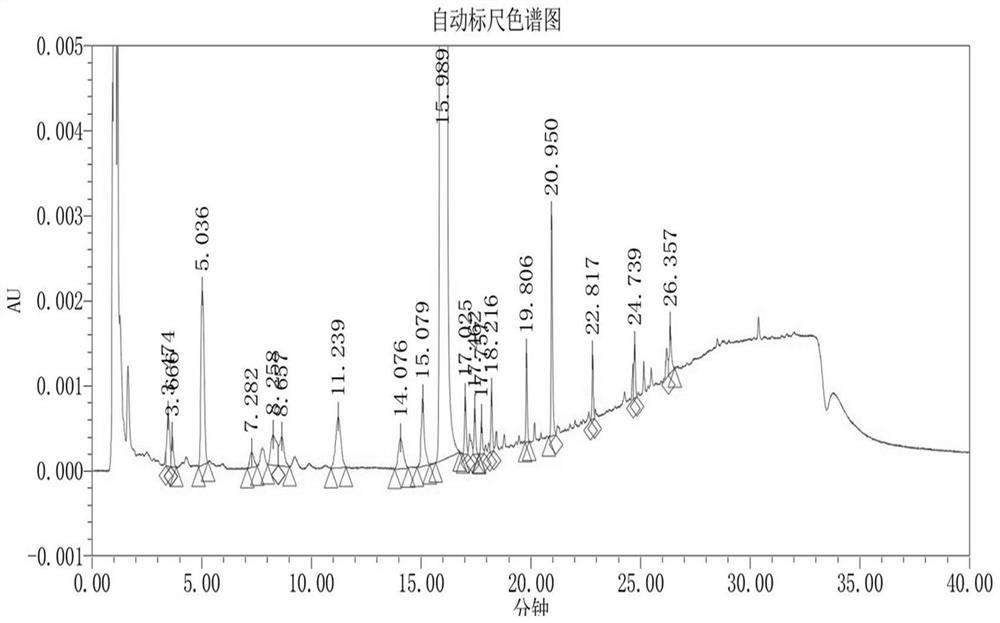
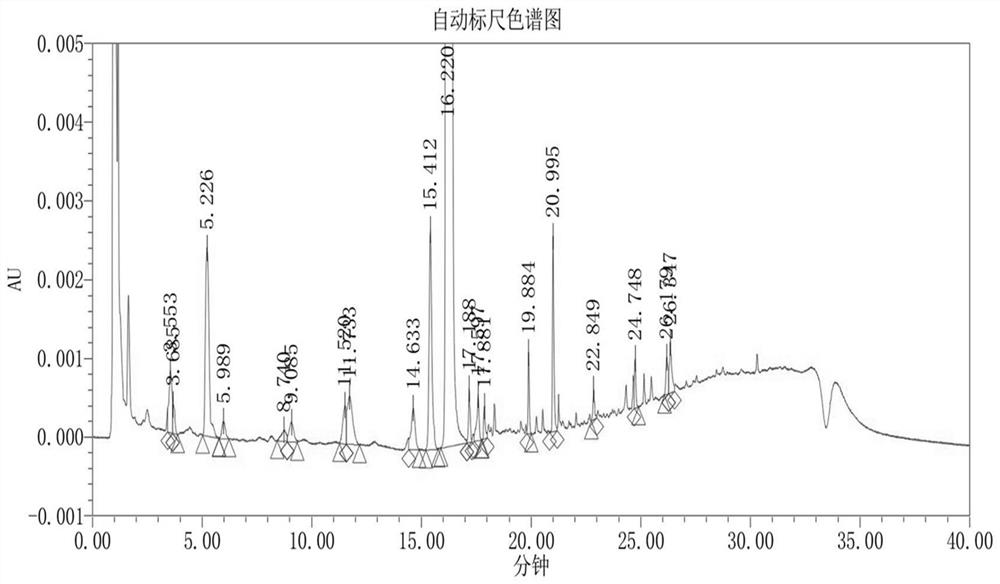
Fluorohydrocortisone acetate tablet and preparation method thereof
A technology of fludrocortisone acetate and fludrocortisone tablets, which is applied in the field of fludrocortisone acetate tablets and its preparation, can solve the problems of increased impurities in the degradation of pine and affect product safety, and avoid negative effects , Improve storage stability, use safety guarantee effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A fludrocortisone acetate tablet of the present invention is mainly composed of fludrocortisone acetate (manufacturer: Henan Lihua Pharmaceutical), lactose (model 11SD, produced by mass ratio of 1:800:150:40:9) supplier: DMF), amylose starch (model Shanjie, manufacturer: Colorcon), talc (manufacturer: Guangxi Longsheng Huamei Talc Development Co., Ltd.) and magnesium stearate (Anhui Shanhe Pharmaceutical Excipients Co., Ltd. ) are prepared.
[0033] The preparation method of the fludrocortisone acetate tablet of the present embodiment comprises the following steps:
[0034] (1) with fludrocortisone acetate and lactose, sieve with 40 mesh sieves, join in mixer;
[0035] (2) adding amylose starch in the mixer and mixing, the control mixing speed is 12rpm, and the one-way mixing is 30min;
[0036] (3) in the material after step (2), add talcum powder and continue to mix, and the control mixing speed is 12rpm, and one-way mixing is 5min;
[0037] (4) in the material afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com