A kind of composition and its preparation method and application
A technology of composition and hydrate, which is applied in the direction of drug combination, pharmaceutical formula, medical preparation of non-active ingredients, etc., can solve the problem of taste masking of the undisclosed dextromethorphan quinidine composition, and achieve the improvement of taste, bitterness and numbing , high safety, and the effect of solving dysphagia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
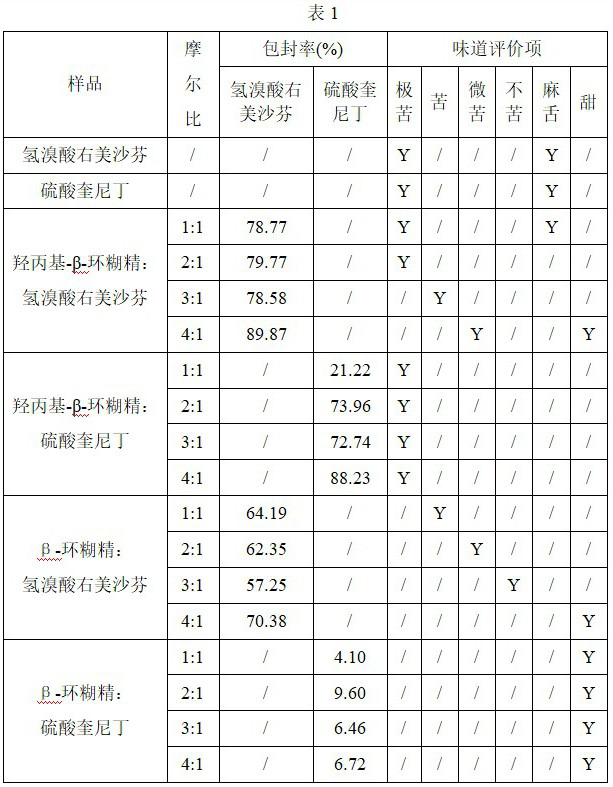
[0029] Preparation of binary inclusion complex by grinding method: weigh an appropriate amount of dextromethorphan hydrobromide and quinidine sulfate, respectively add cyclodextrin, add an appropriate amount of purified water, grind, and dry in an oven at 60 °C. The cyclodextrin-saturated ethanol solution was washed, dried in a 60° C. oven again, ground and sieved to obtain dextromethorphan cyclodextrin inclusion complex and quinidine cyclodextrin inclusion complex, respectively.
[0030] The encapsulation efficiency of the obtained inclusion compound was detected, and the taste-masking effect of cyclodextrin was evaluated by artificial taste method. The results are shown in Table 1.
[0031] Artificial taste method: The volunteers did not eat, drink, or chew gum at least 1 hour before the test, and rinsed their mouths with deionized water more than 3 times. The volunteers put the sample in the mouth, spit it out after 10s, rinsed the mouth with deionized water until there was...
Embodiment 2
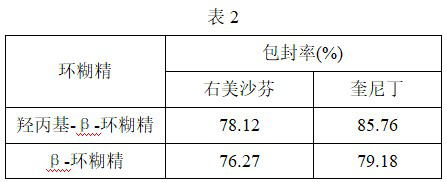
[0035] Preparation of ternary inclusion compound by grinding method: after weighing dextromethorphan hydrobromide and quinidine sulfate, mix, adopt the preparation method in Example 1 to prepare cyclodextrin, dextromethorphan hydrobromide and quinidine sulfate Dextromethorphan quinidine cyclodextrin inclusion complex in a molar ratio of 3:1:0.236. The encapsulation efficiency of dextromethorphan quinidine inclusion complexes prepared from different cyclodextrins was detected, and the results are shown in Table 2.
[0036]
[0037]The results showed that the encapsulation efficiency of dextromethorphan and quinidine by the ternary inclusion complex was better. Compared with the binary clathrate, the ternary clathrate significantly improved the encapsulation efficiency of dextromethorphan and quinidine. In particular, the ternary inclusion complex of β-cyclodextrin can even increase the encapsulation efficiency of quinidine by more than 10 times.
Embodiment 3
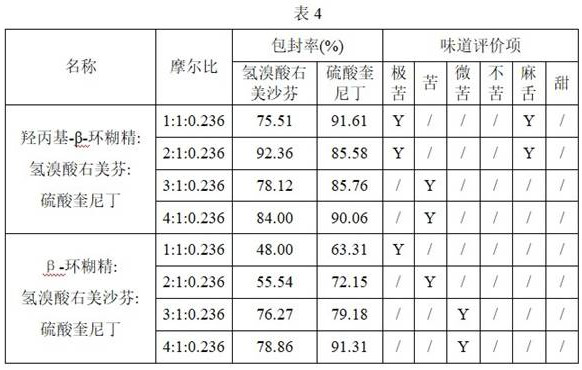
[0039] Aqueous solution method to prepare ternary inclusion complex: weigh an appropriate amount of cyclodextrin into a beaker, add dextromethorphan hydrobromide and quinidine sulfate to make cyclodextrin, dextromethorphan hydrobromide and quinidine sulfate molar The ratio is 3:1:0.236, an appropriate amount of deionized water is added, and the mixture is stirred at 60° C. until the mixture is completely dissolved, and the solution is freeze-dried to obtain a freeze-dried powder. The obtained freeze-dried powder was washed with a saturated ethanol solution of cyclodextrin, filtered and then dried in an oven at 60° C., ground, crushed and sieved to obtain a dextromethorphan quinidine inclusion complex. The encapsulation efficiency of dextromethorphan quinidine inclusion complexes prepared from different cyclodextrins was detected, and the results are shown in Table 3.
[0040]
[0041] The results show that the encapsulation efficiency of dextromethorphan and fenquinidine is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com