Molecular marker composition for diagnosing tuberculous pleurisy and application thereof
A technology of tuberculous pleurisy and molecular markers, applied in the field of medicine, can solve the problems that patients cannot obtain diagnosis results, cannot be detected, and limited diagnostic techniques
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] miRNA microarray screening test
[0055] (1) Research objects
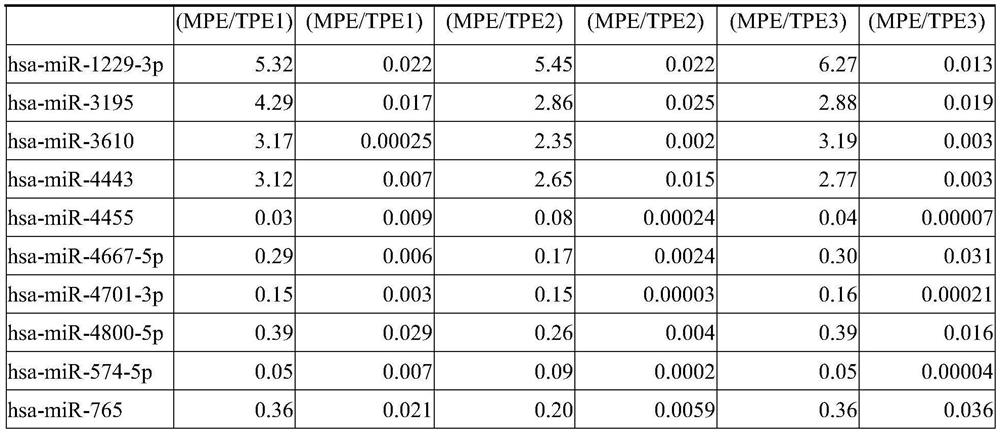
[0056] Patients with tuberculous pleurisy: Three categories of patients with tuberculous pleurisy were included according to the level of clinical evidence,
[0057] Group 1 (TPE1): patients with positive pleural effusion for Mycobacterium tuberculosis etiology;
[0058] Group 2 (TPE2): patients with pleural effusion negative for Mycobacterium tuberculosis pathogenic test but positive for sputum specimen pathogenic test;
[0059] Group 3 (TPE3): Both pleural effusion and sputum Mycobacterium tuberculosis pathogenic tests were negative, but the clinical diagnosis was tuberculous pleurisy.
[0060] Inclusion criteria for patients with tuberculous pleurisy:
[0061] ① There are clinical symptoms such as low fever, night sweats, weight loss, chest pain, dry cough; ② The pleural effusion is consistent with the change of exudate; ③ After anti-tuberculosis treatment, the pleural effusion is absorbed and the cli...
Embodiment 2
[0075] qPCR validation test
[0076] (1) Reagents:
[0077] Internal reference gene for qPCR: Cel-miR-39 (QIAGEN, Germany);
[0078] The reagents for RNA extraction, reverse transcription and qPCR detection were commercial reagents (QIAGEN, Germany).
[0079] (2) Primers:
[0080] miRNA upstream primer
[0081] Cel-miR-39: 5'-GCCGAGAGCTGATTTCGTCT-3'
[0082] hsa-miR-574-5p: 5'-TCGGCAGGTGAGTGTGTGT-3'
[0083] hsa-miR-4455: 5’-GCCGAGAGGGTGTGTGTGTT-3’
[0084] hsa-miR-4701-3p: 5’-TCGGCAGGATGGGTGATG-3’
[0085] The downstream primer is a universal primer: 5'-CTCAACTGGTGTCGTGGA-3'
[0086] (3) Instrument: ABI Quantistudio7.
[0087] (4) Test method:
[0088] The above 10 miRNAs were subjected to a large-scale qPCR validation test (188 patients with tuberculous pleurisy and 122 patients with malignant pleural disease).
[0089] The specific experimental process is as follows:
[0090] 1) RNA extraction: Take 250 μL of pleural effusion, centrifuge at 4°C, 16000×g for 10 mi...
Embodiment 3
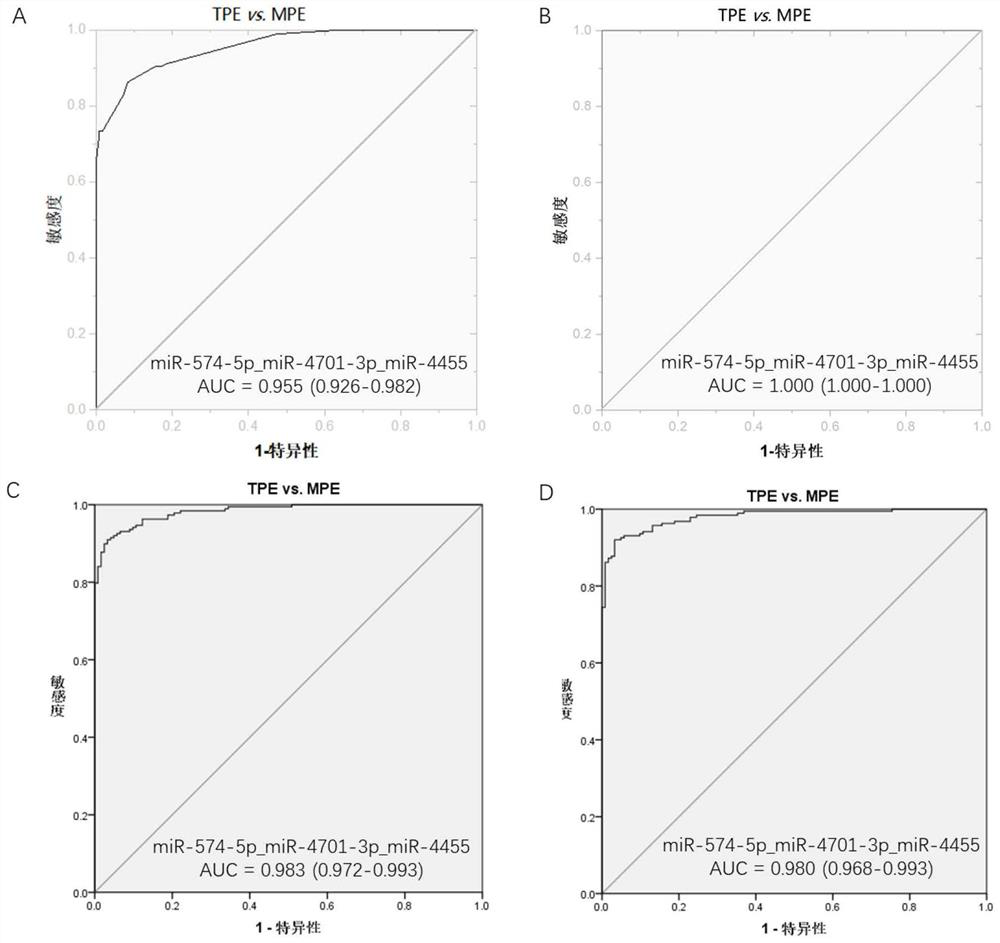
[0106] Diagnostic Sensitivity and Specificity Analysis of ROC Analysis of Single miRNA
[0107] Three differential miRNAs, miR-574-5p, miR-4701-3p, and miR-4455 in pleural effusion, were analyzed using receiver operating characteristic (ROC) curves in 188 patients with tuberculous pleurisy and 122 patients with malignant pleural disease. The ROC curve analysis was carried out, and the results are shown in Table 4.
[0108] Table 4. Diagnostic value of a single miRNA in pleural effusion for differential diagnosis of tuberculous pleurisy and malignant pleural disease
[0109]
[0110] The results showed that the area under the ROC curve (AUC) of miR-574-5p, miR-4701-3p and miR-4455 for the differential diagnosis of tuberculous pleurisy and malignant pleural disease were all greater than 0.9, which had a good differential diagnosis of tuberculous pleurisy and malignant pleural disease. Capacity for malignant pleural disease.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com