Use of neutrophil elastase inhibitors in lung diseases
An anti-trypsin, lung disease technology, applied in the field of chronic lung disease, can solve problems affecting the quality of life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
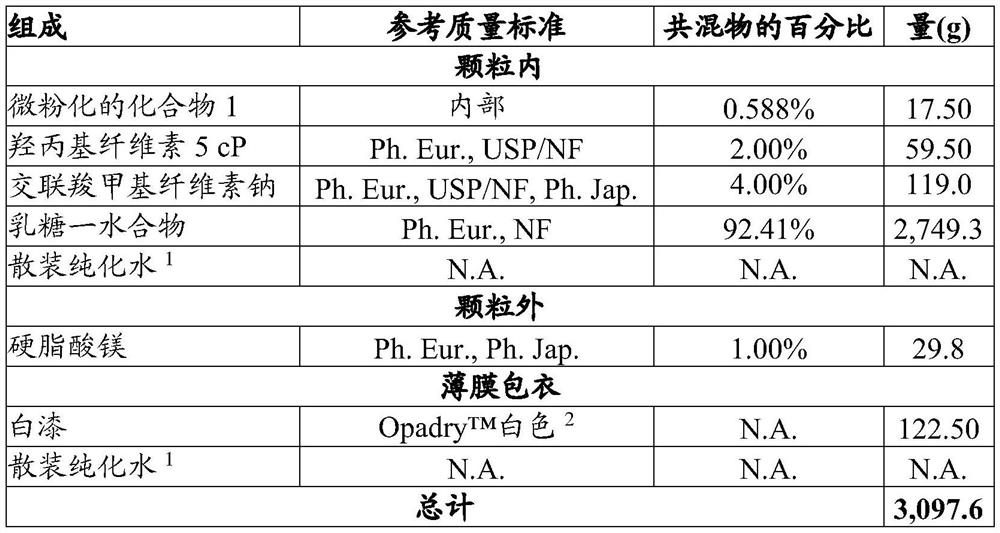
[0077] Example 1. Preparation of tablets.
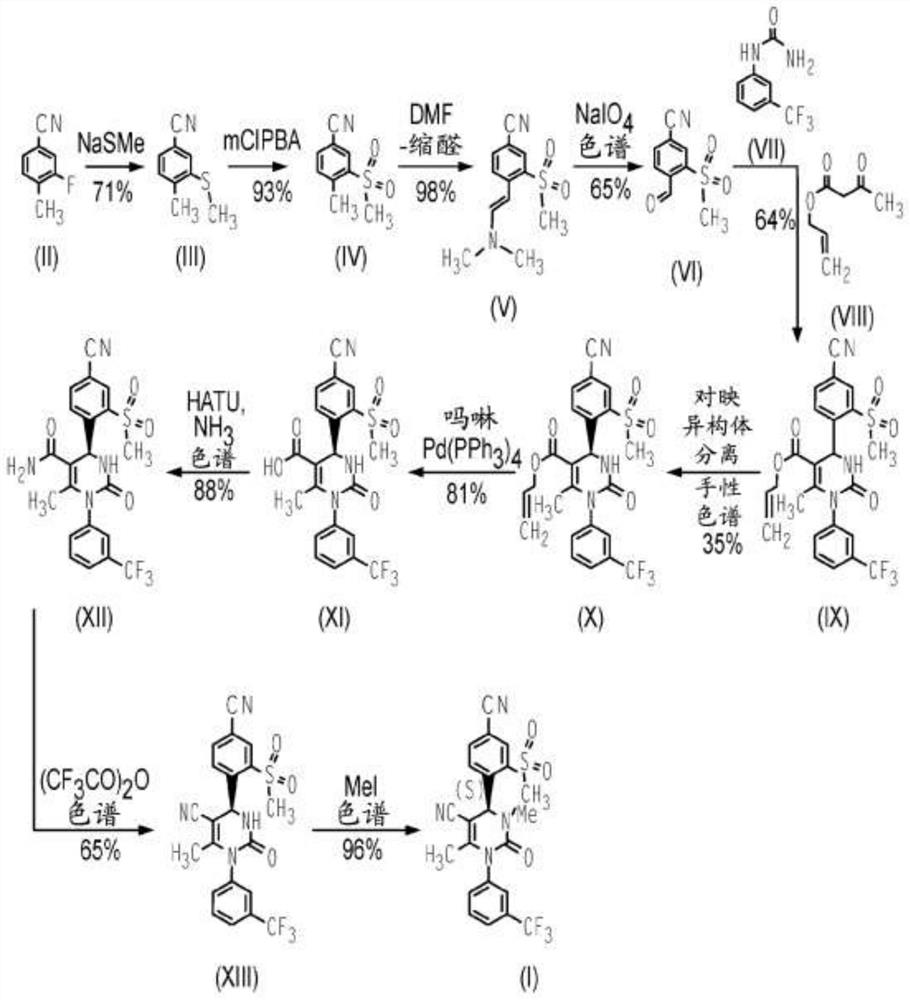
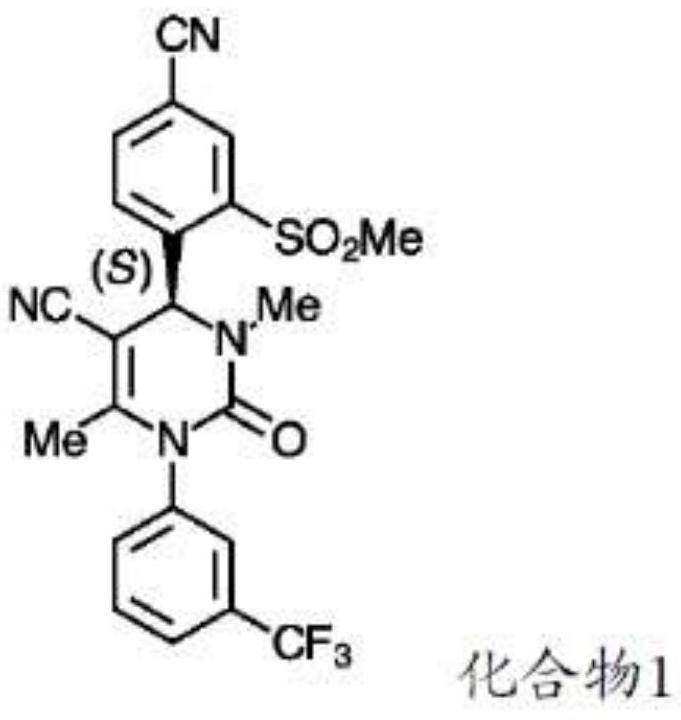
[0078] Compound 1(4S)-4-[4-cyano-2-(methylsulfonyl)phenyl]-3,6-dimethyl-2-oxo-1-[3-(trifluoromethyl) yl)phenyl]-1,2,3,4-tetrahydropyrimidine-5-carbonitrile is formulated into tablets for oral use. These tablets are manufactured using standard pharmaceutical processing techniques. All inactive pharmaceutical ingredients in the following examples meet the requirements of the United States Pharmacopoeia (USP), National Formulary (NF), European Pharmacopoeia (Ph. Eur.) and / or Japanese Pharmacopoeia (Ph. Jap.) as indicated, And tested and released according to the monograph for each ingredient specified in the indicated standard. Batch sizes vary depending on the amount required for a specific clinical purpose. The following two examples show the qualitative / quantitative composition of exemplary doses and are for illustrative purposes. It should be understood that additional dose sizes and batch sizes are contemplated by the present i...
Embodiment 2
[0095] Example 2. Phase 1 clinical study of compound 1 in healthy patients
[0096] Study description. Phase 1, single-center, randomized, double-blind, placebo-controlled, single-ascending-dose study designed to evaluate the safety, tolerability, and pharmacokinetics (PK) of Compound 1 in healthy subjects. Good Clinical Practice (GCP), ethical principles derived from the Declaration of Helsinki and all other applicable laws, rules and regulations.
[0097] Within each dose cohort, subjects were randomized in a 3:1 ratio (6 active agents and 2 placebo) to receive Compound 1 or placebo. Following screening, subjects received a single dose of study drug and were monitored during the clinical phase and outpatient follow-up. Subjects were confined to the study site on study days -2 to 7 for the collection of PK and safety assessments. After leaving the study site on study day 7, subjects returned to the study site on study days 14, 21, 28 and 35.
[0098] result. A total of...
Embodiment 3
[0101] Example 3. Phase 2 clinical study of compound 1 in patients with AATD
[0102] Study description . This study is a Phase 2, multicenter, double-blind, randomized (1:1), placebo-controlled, proof-of-concept study to evaluate compound 1 administered daily for 12 weeks in patients with confirmed AATD (α-1ZZ Safety and tolerability in patients with genotype [Pi*ZZ]) or alpha-1 null phenotype [Pi* null phenotype], AAT level <11 μM (0.5g / L)) and AATD-related emphysema properties and effects on pharmacodynamic markers. Trials were conducted in accordance with Good Clinical Practice (GCP), ethical principles derived from the Declaration of Helsinki and all other applicable laws, rules and regulations. Eligible patients will be enrolled and randomized in a 1:1 ratio (1 active agent and 1 placebo) within 30 days of screening to receive Compound 1 at 20 mg per day or 10 mg per day or matching placebo per day for 84 days (12 weeks). Compound 1 will be provided as immediate re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com