Patents
Literature
43 results about "Trypsin deficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Also called AAT deficiency, alpha-1 antitrypsin deficiency is a genetic disease, which means it's passed down from your parents. It can cause serious lung disease that makes it hard to breathe. It can also cause liver disease that leads to jaundice, which makes your skin look yellowish.
Raav-based compositions and methods for treating alpha-1 Anti-trypsin deficiencies
ActiveUS20160326524A1Improve the level ofToxic reductionOrganic active ingredientsPeptide/protein ingredientsDiseaseTrypsin deficiency
Owner:UNIV OF MASSACHUSETTS +1
Raav-based compositions and methods for treating alpha-1 Anti-trypsin deficiencies
ActiveUS20160186211A1Improve the level ofToxic reductionOrganic active ingredientsBiocideDiseaseTrypsin deficiency
Owner:UNIV OF MASSACHUSETTS +1
Method for treating pulmonary diseases using rho kinase inhibitor compounds
InactiveUS20100204210A1Not effectiveBiocidePharmaceutical delivery mechanismDiseaseOccupational pulmonary disease
This invention relates to methods of treating pulmonary diseases in patients that beta adrenergic receptor agonist therapy is not effective. The method comprises the steps of: identifying a patient who suffers from a pulmonary disease and has reduced responsiveness to treatment with one or more beta adrenergic receptor agonists, and administering to the patient an effective amount of a Rho kinase inhibitor compound, wherein said pulmonary disease is selected from the group consisting of: asthma, chronic obstructive pulmonary disease, respiratory tract illness caused by respiratory syncytial virus infection such as RSV-induced wheezing, airway hyperreactivity, or bronchiolitis, bronchiectasis, alpha-1-antitrypsin deficiency, lymphangioleiomyomatosis, cystic fibrosis, bronchiolitis or wheezing caused by agents other than respiratory syncytial virus, chronic bronchitis, and occupational lung diseases.
Owner:INSPIRE PHARMA
Novel genes, compositions, and methods for modulating the unfolded protein response
InactiveUS20050250182A1Increased transactivation potentialIncrease volumeSugar derivativesHydrolasesAutoimmune conditionAutoimmune disease
The present invention relates to methods and compositions for modulating the unfolded protein response. The method further relates to methods and compositions for the treatment and diagnosis of protein conformational diseases or disorders, including, but not limited to, α1-antitrypsin deficiency, cystic fibrosis, and autoimmune diseases and disorders. The invention further provides methods for modulating the unfolded protein response by modulating XBP1 mRNA splicing.
Owner:UNIV OF MICHIGAN THE
Materials and methods for treatment of alpha-1 antitrypsin deficiency
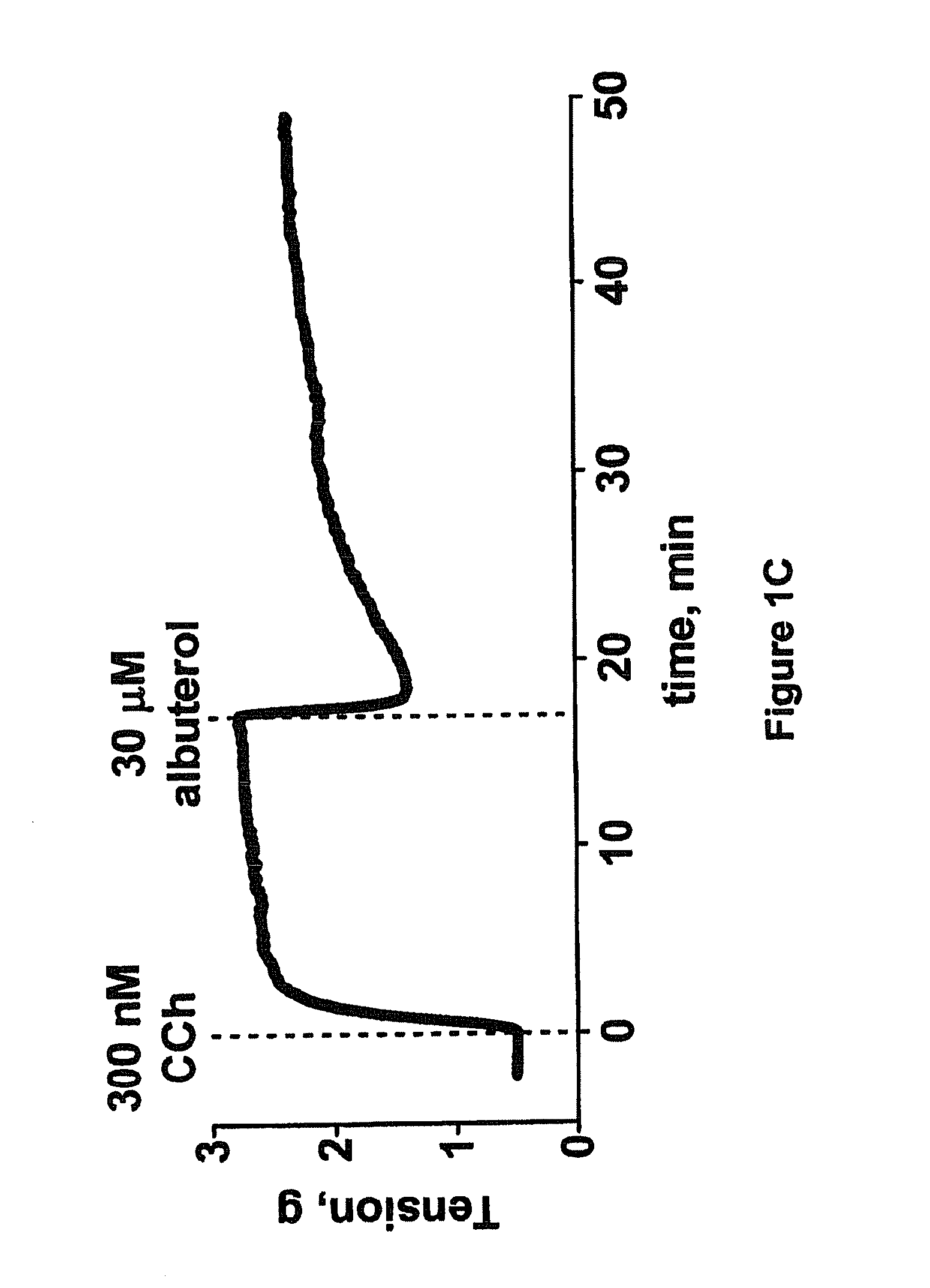
PendingUS20190010490A1Easy to insertOrganic active ingredientsSpecial deliveryGenome editingTrypsin deficiency
Owner:CRISPR THERAPEUTICS AG
Small compounds that correct protein misfolding and uses thereof
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Chimeric dystrophin proteins to treat dystrophinopathies
InactiveUS20150329609A1SsRNA viruses negative-sensePolypeptide with localisation/targeting motifMelanomaMarfan syndrome
A chimeric protein that is a fusion construct of a series of functional domains is used to deliver a therapeutic agent to a human subject suffering from disease. In some embodiments, the chimeric protein includes a therapeutic region and a transportation region. The transportation region allows the chimeric protein to be moved across a cellular membrane of an affected cell within the subject. The therapeutic region can be effective in the treatment of, for example, muscular dystrophy, diastrophic dysplasia, malignant melanoma, porphyria, alpha-1 antitrypsin deficiency, Aicardi-Goutieres syndrome, cystic fibrosis, progeria, Marfan syndrome, tuberous sclerosis, adrenoleukodystrophy, and the like.
Owner:SERENDIPITY BIOTECH INC
Modulators of alpha-1 antitrypsin
Owner:VERTEX PHARMA INC
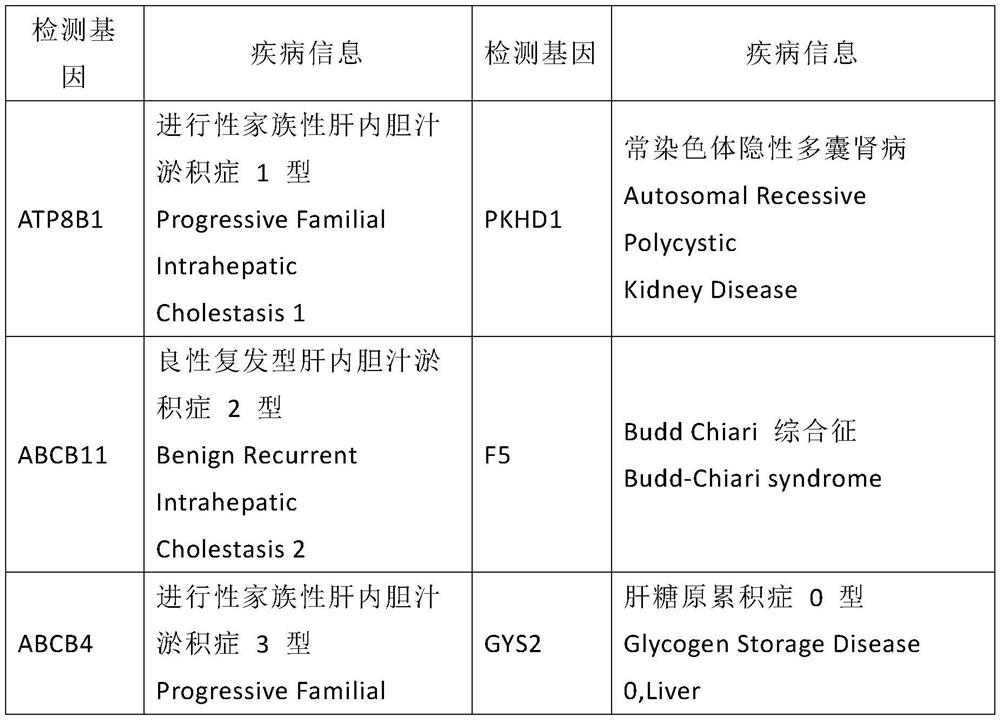
Kit used for screening genetic liver diseases
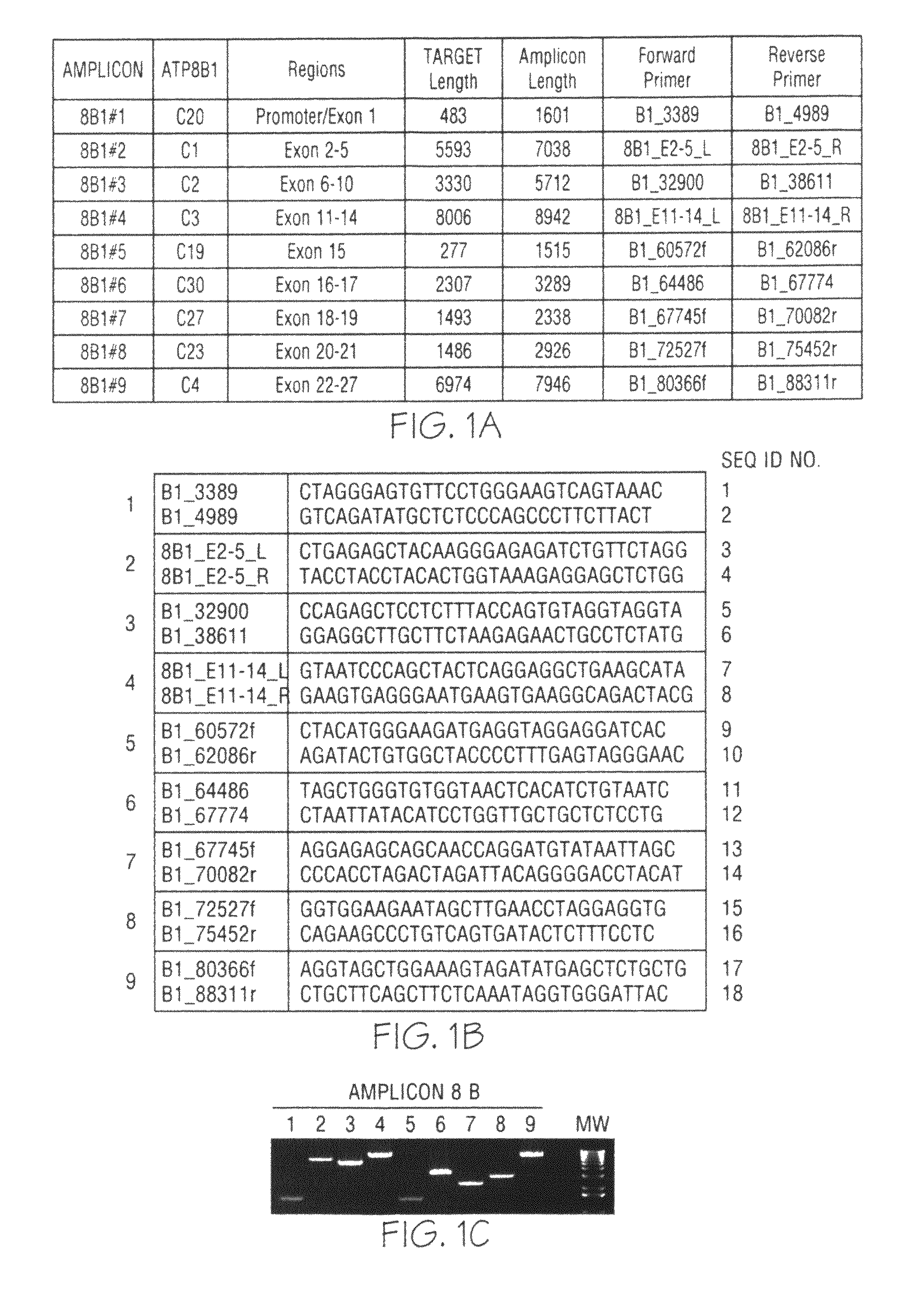
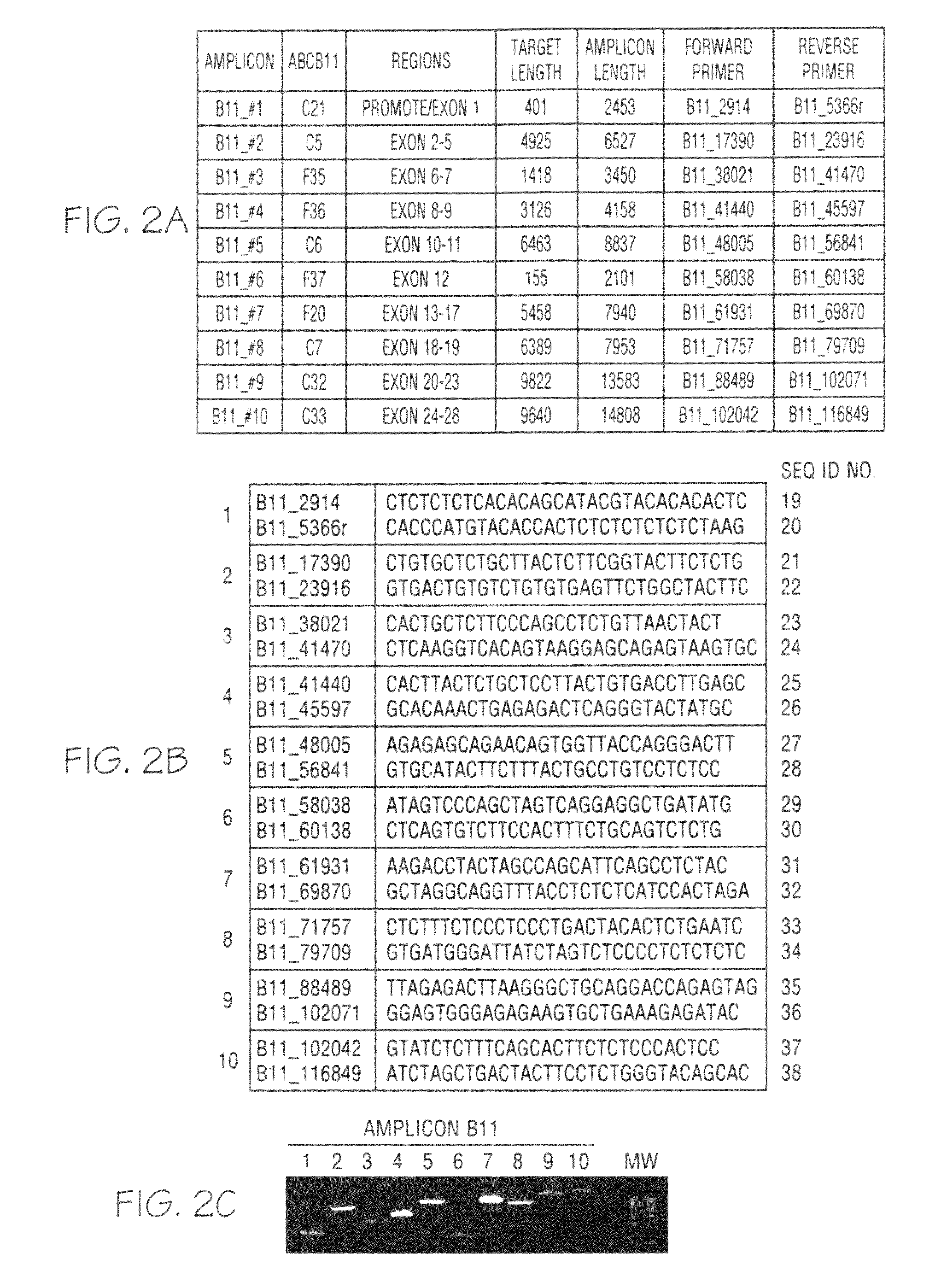
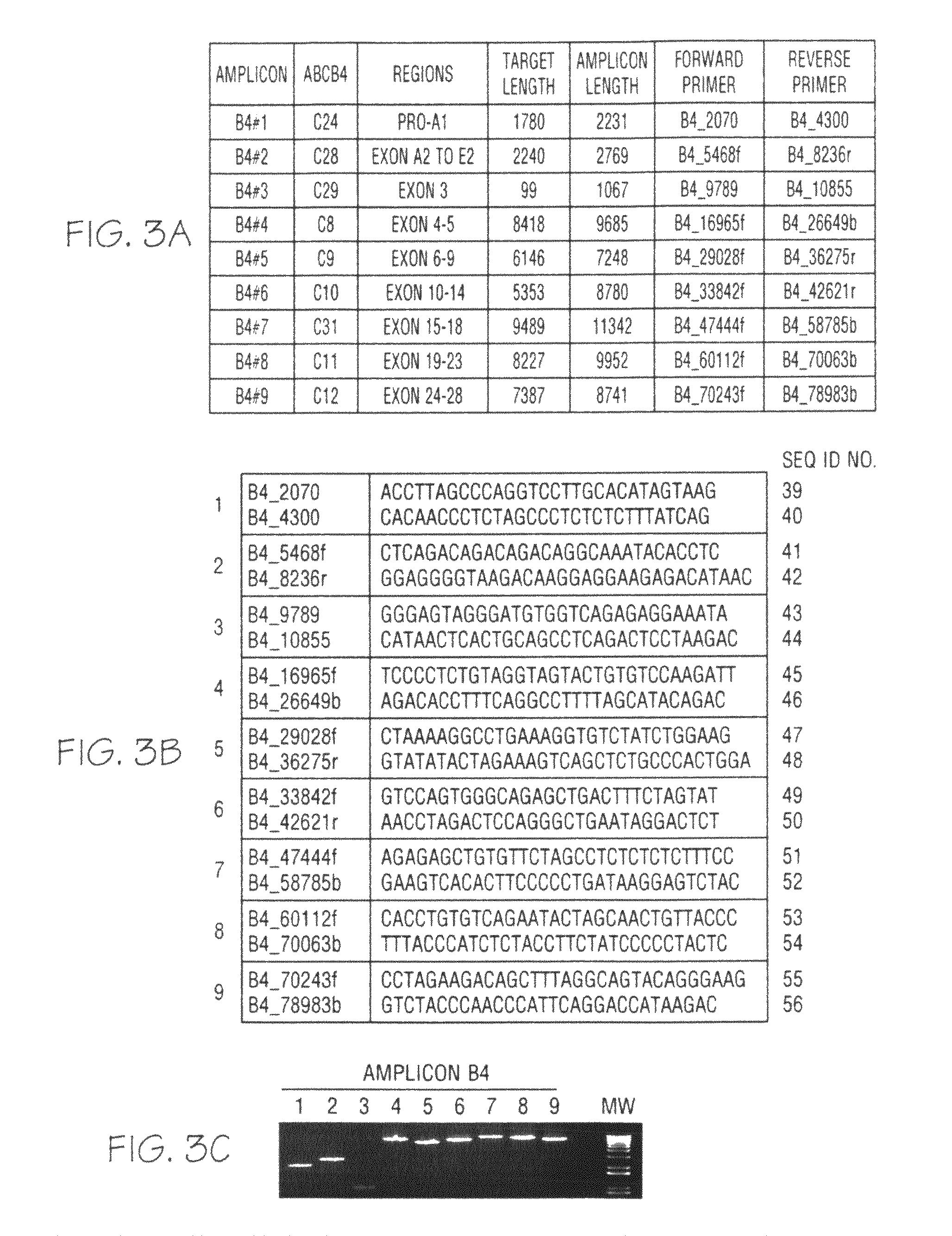
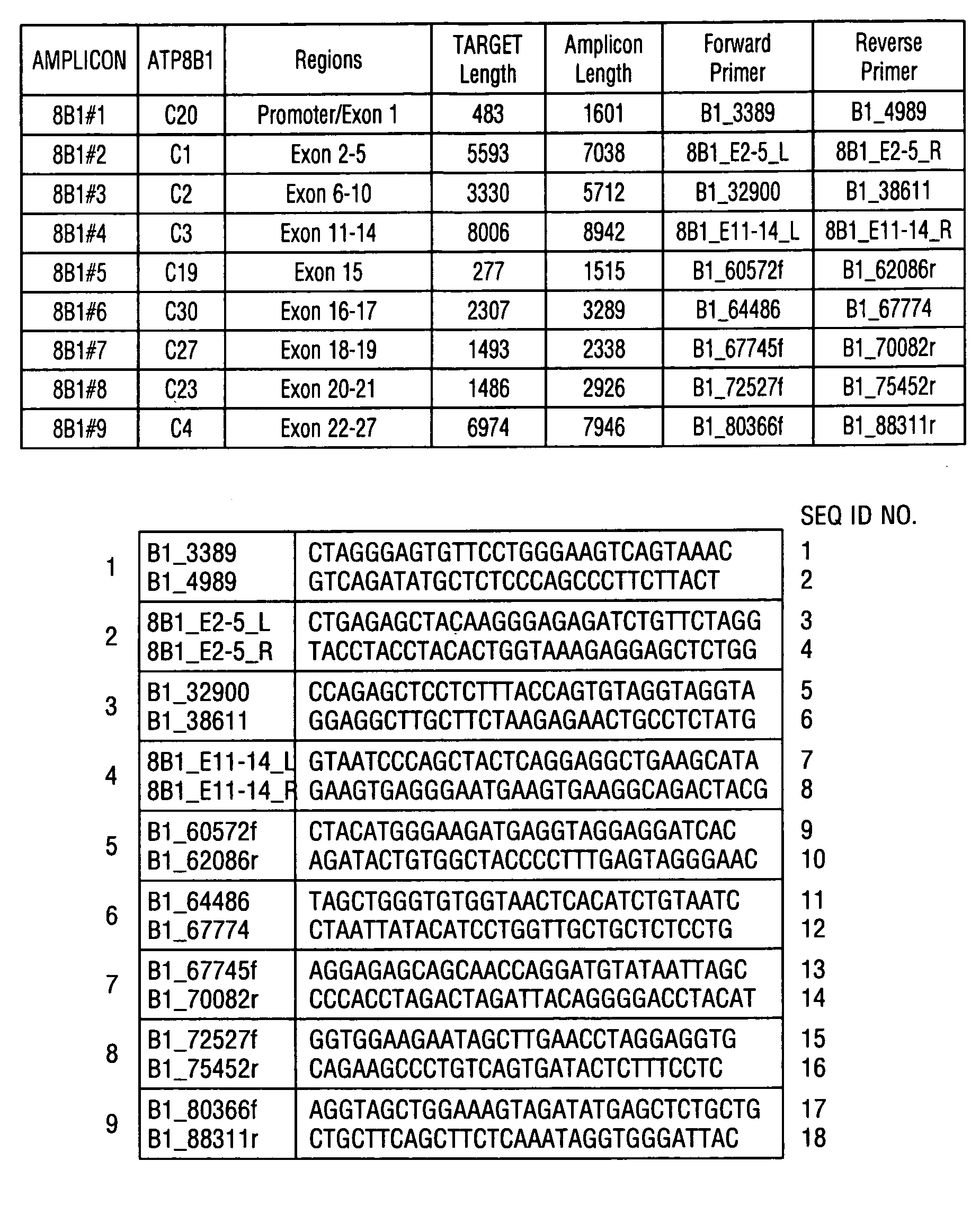
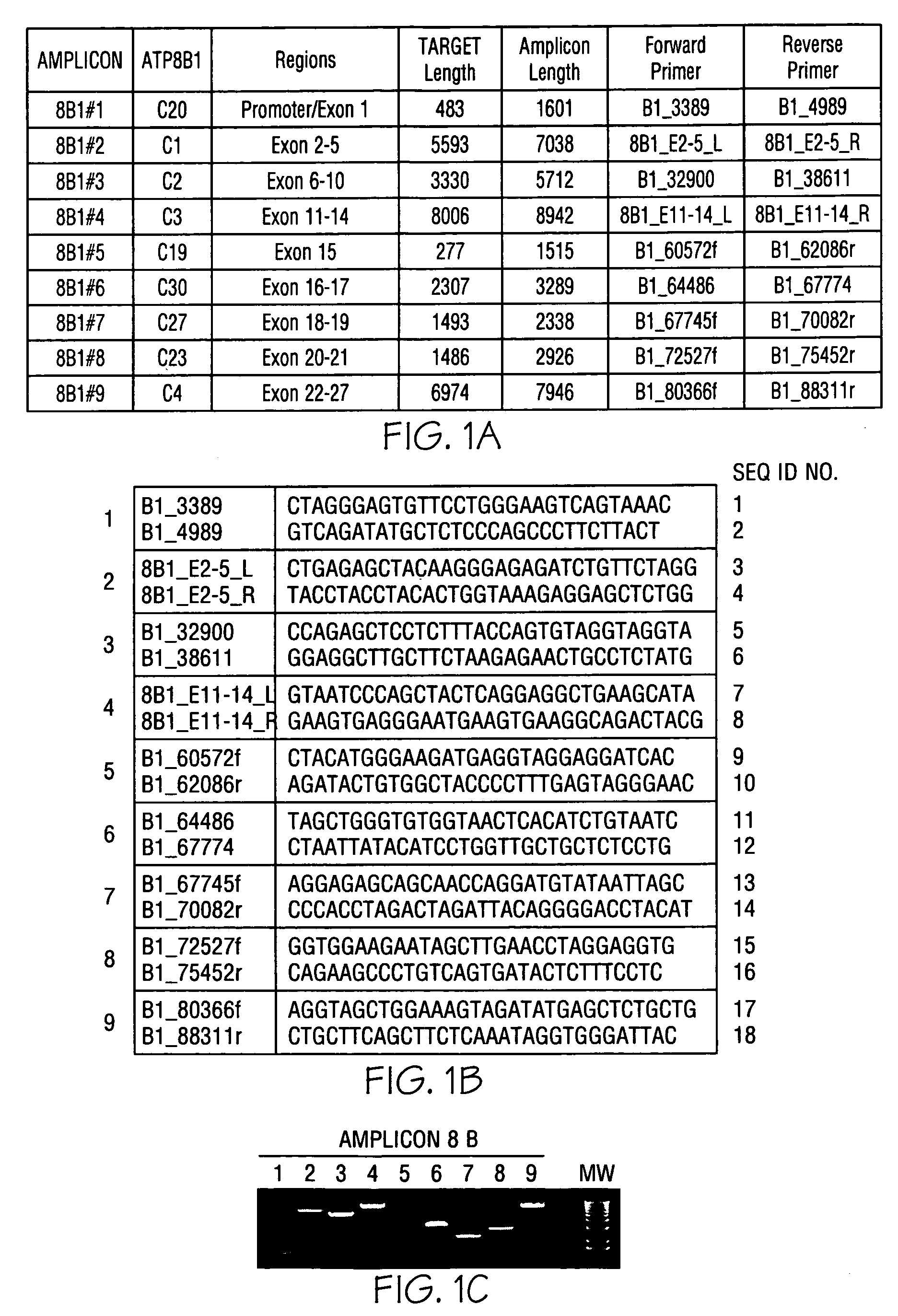
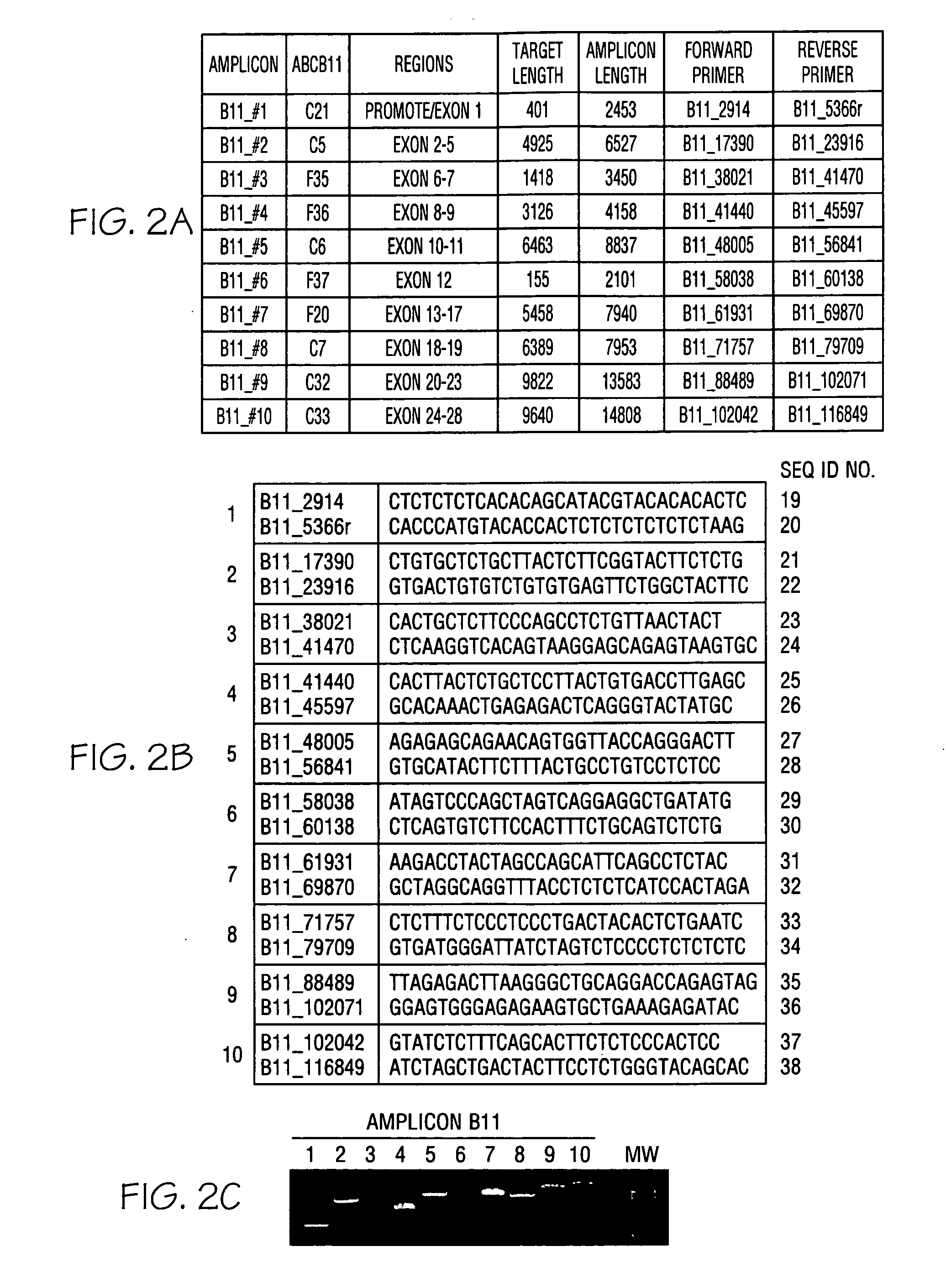
ActiveCN108913761AInterpretation is clear and objectiveHigh detection throughputMicrobiological testing/measurementNPC1Autosomal Recessive Polycystic Kidney Disease
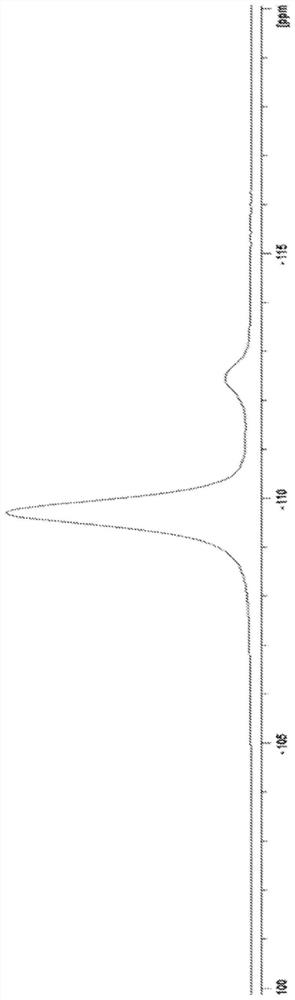
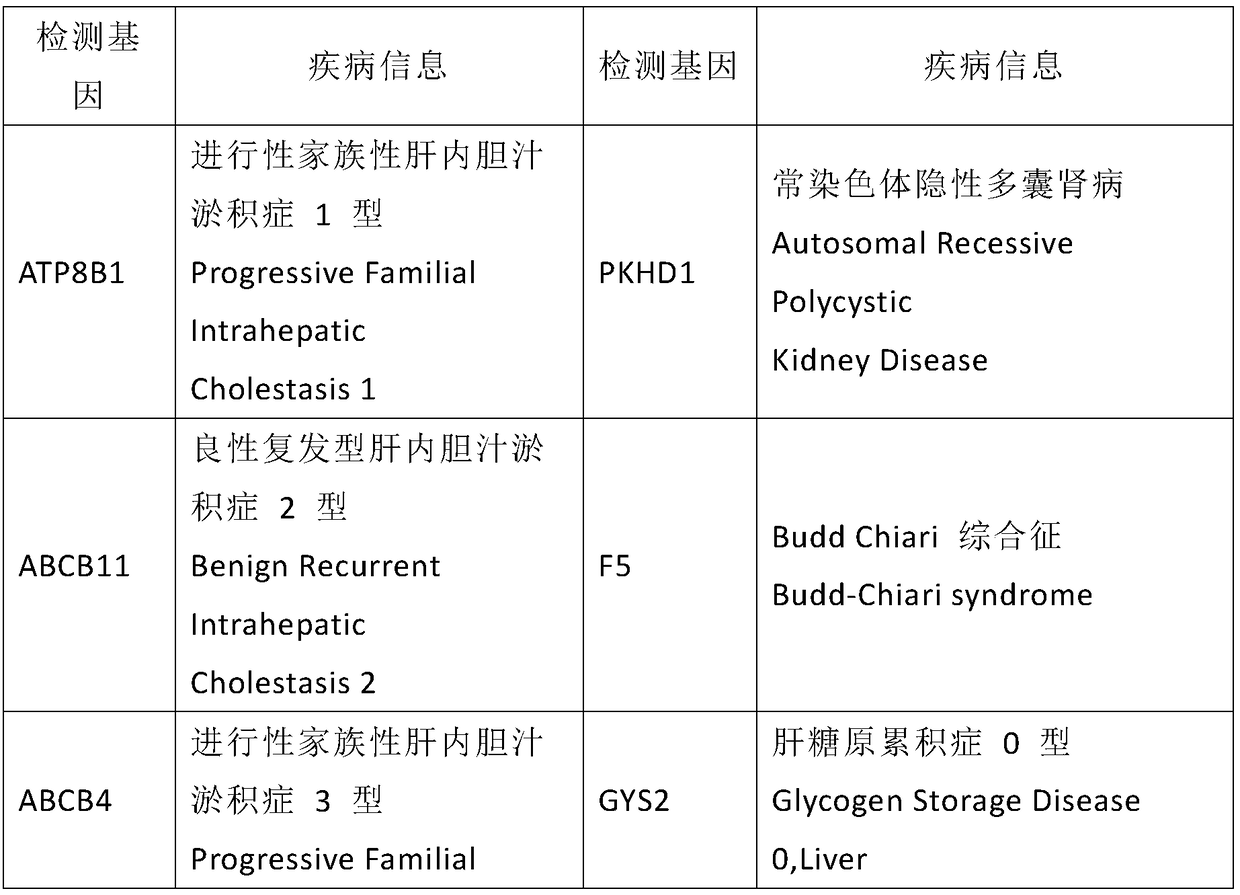
The invention discloses a kit used for screening genetic liver diseases. The kit is high in detection flux, sensitivity, and specificity. The genetic liver diseases comprise progressive familial intrahepatic cholestasis, benign recurrent intrahepatic cholestasis, congenital bile acid synthesis defect, idiopathic bile acid malabsorption, Dubin-Johnson syndrome, hereditary hemochromatosis, alpha1 antitrypsin deficiency, glycogen storage disease, autosomal recessive polycystic kidney disease, Budd-Chiari syndrome, and the like. 39 genes, including ATP8B1, ABCB11, ABCB4, TJP2, NR1H4, HSD3B7, AKR1D1, CYP7B1, AMACR, ABCD3, SLC10A2, ABCC2, HFE, TFR2, SERPINA1, G6PC, SLC37A4, AGL, PYGL, SLC40A1, PKHD1, F5, GYS2, VIPAS39, SLC25A13, JAG1, NOTCH2, PHKA2, PHKB, PHKG2, PHKA1, ALAD, HAMP, HFE2, SMPD1, ATP7B, ABCA1, NPC2, and NPC1, are detected in screening of genetic liver diseases. The kit comprises targeting capture probes SEQ NO:1-SEQ NO:722 targeting at all the exons of the 39 genes, and the kitis used for gene detection.
Owner:施军平 +1
Classification and diagnosis of the molecular basis of cholestasis
InactiveUS7847088B2Sugar derivativesMicrobiological testing/measurementNucleotideNucleotide sequencing
The methods and compositions of the invention find use in the clinical diagnosis of cholestasis related syndromes, particularly PFIC types 1, 2, and 3; BRIC types 1 and 2; Alagille syndrome, and alpha1-antitrypsin deficiency. The compositions of the invention include isolated nucleic acid molecules and oligonucleotide pairs suitable for use in amplifying regions of cholestasis related genes. Compositions of the invention include a cholestasis related gene resequencing microarray suitable for determining the nucleotide sequence of a region of a cholestasis related gene. Knowledge of the nucleotide sequence of one or more regions of a patient's cholestasis related gene allows diagnosis of the patient's syndrome.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
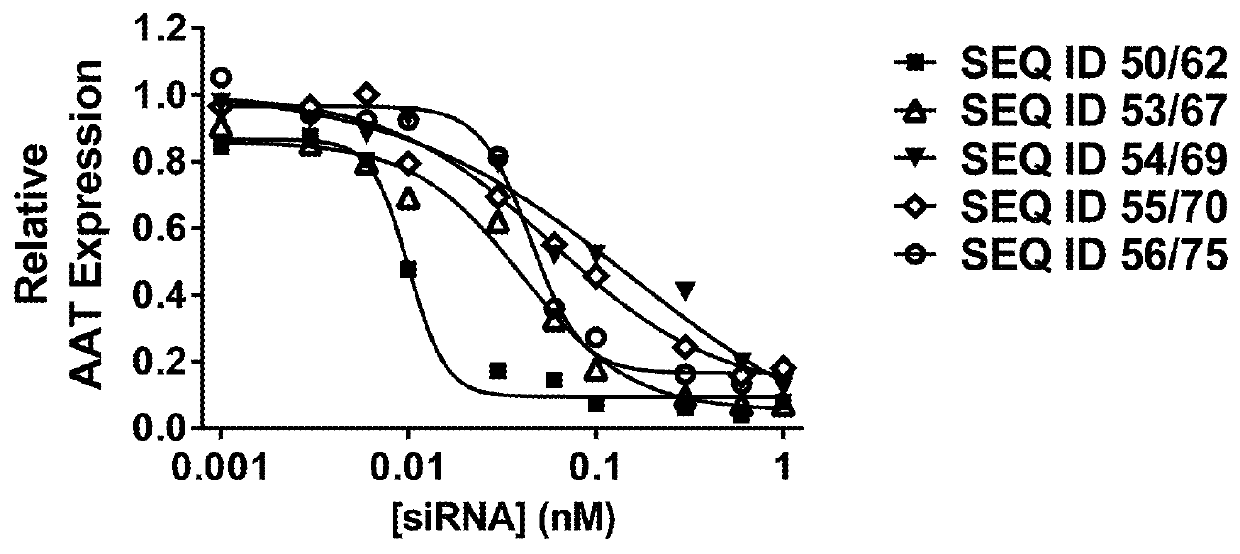
Compositions and methods for inhibiting gene expression of alpha-1 AntiTrypsin
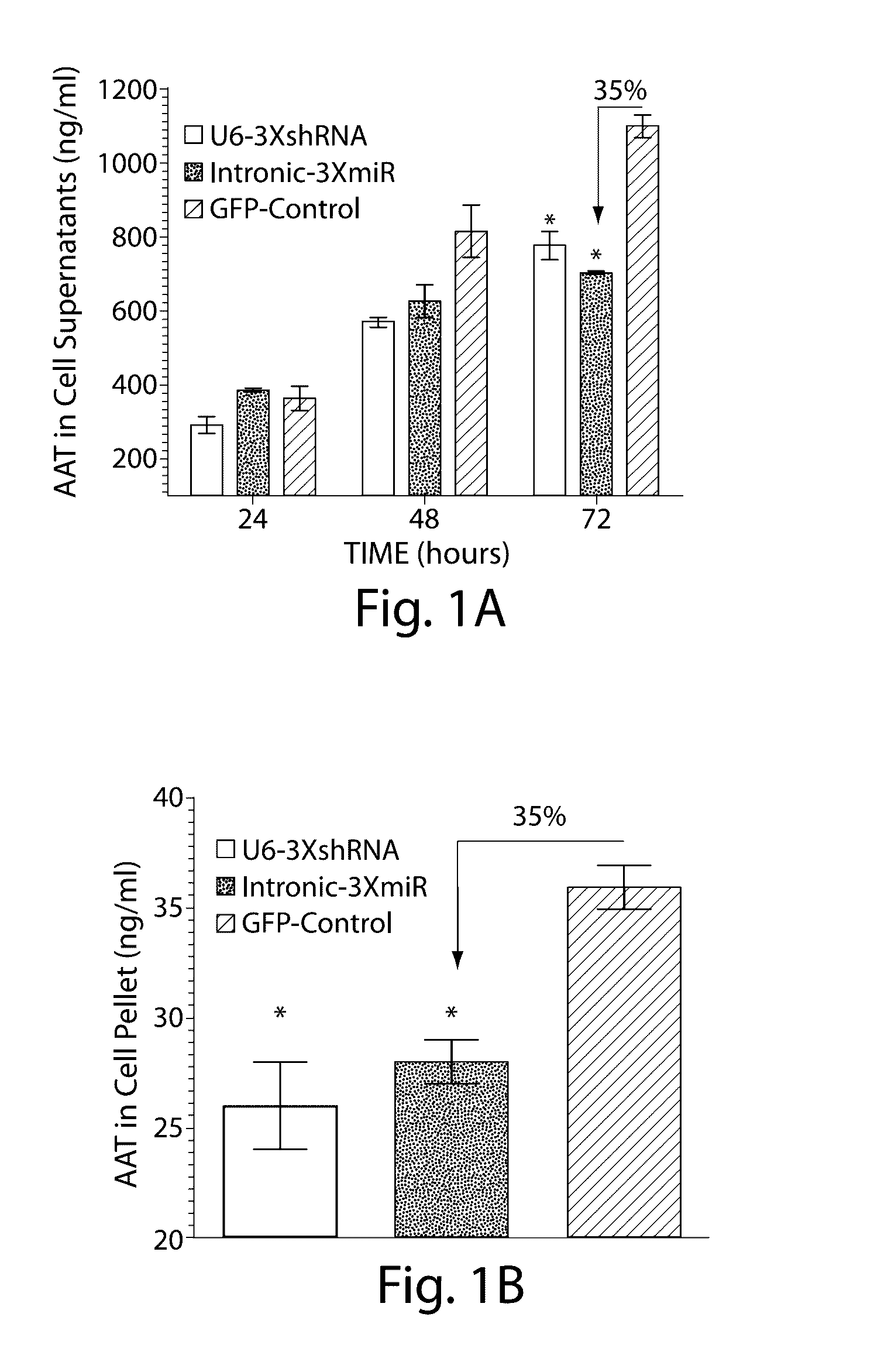
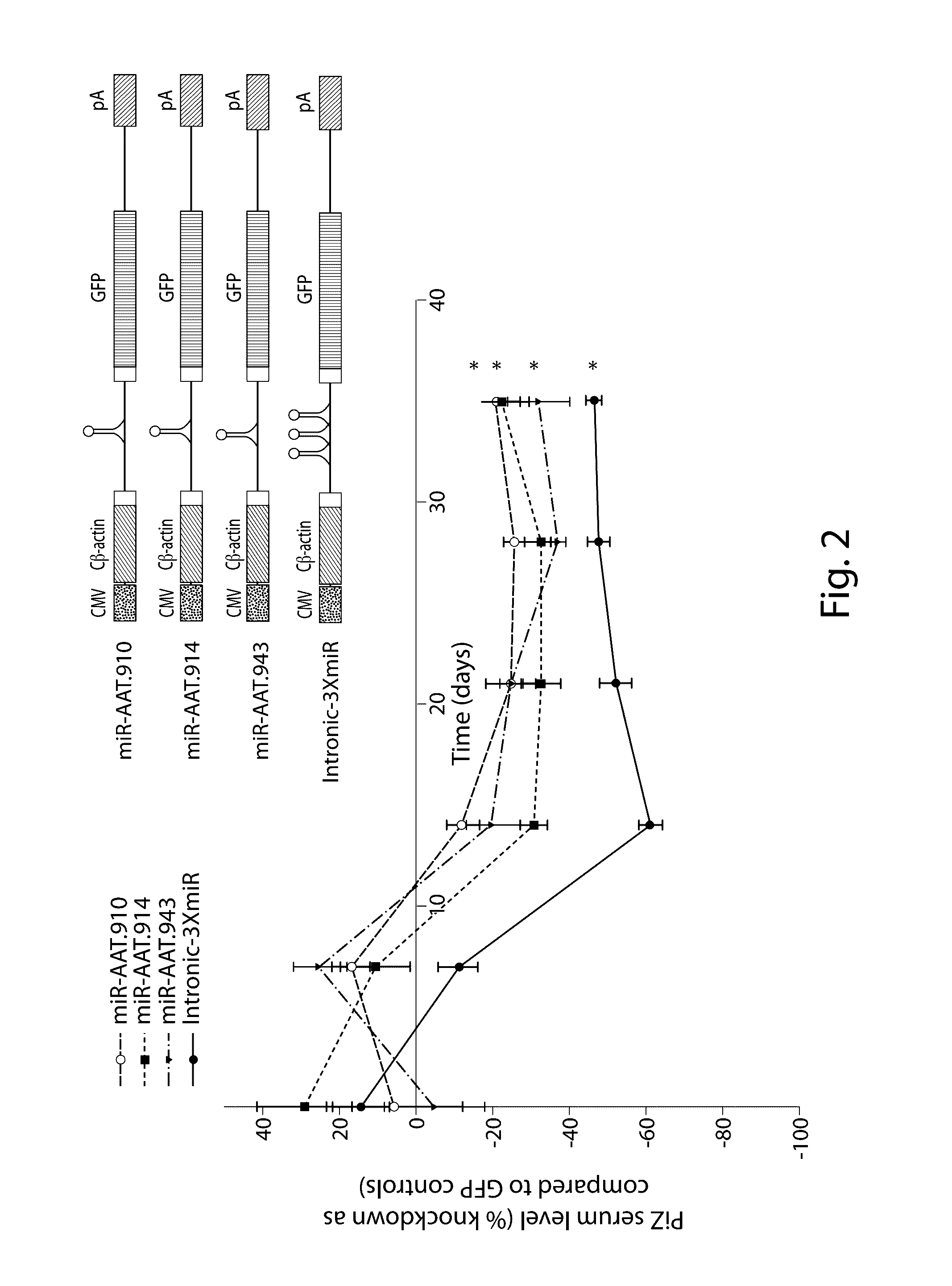
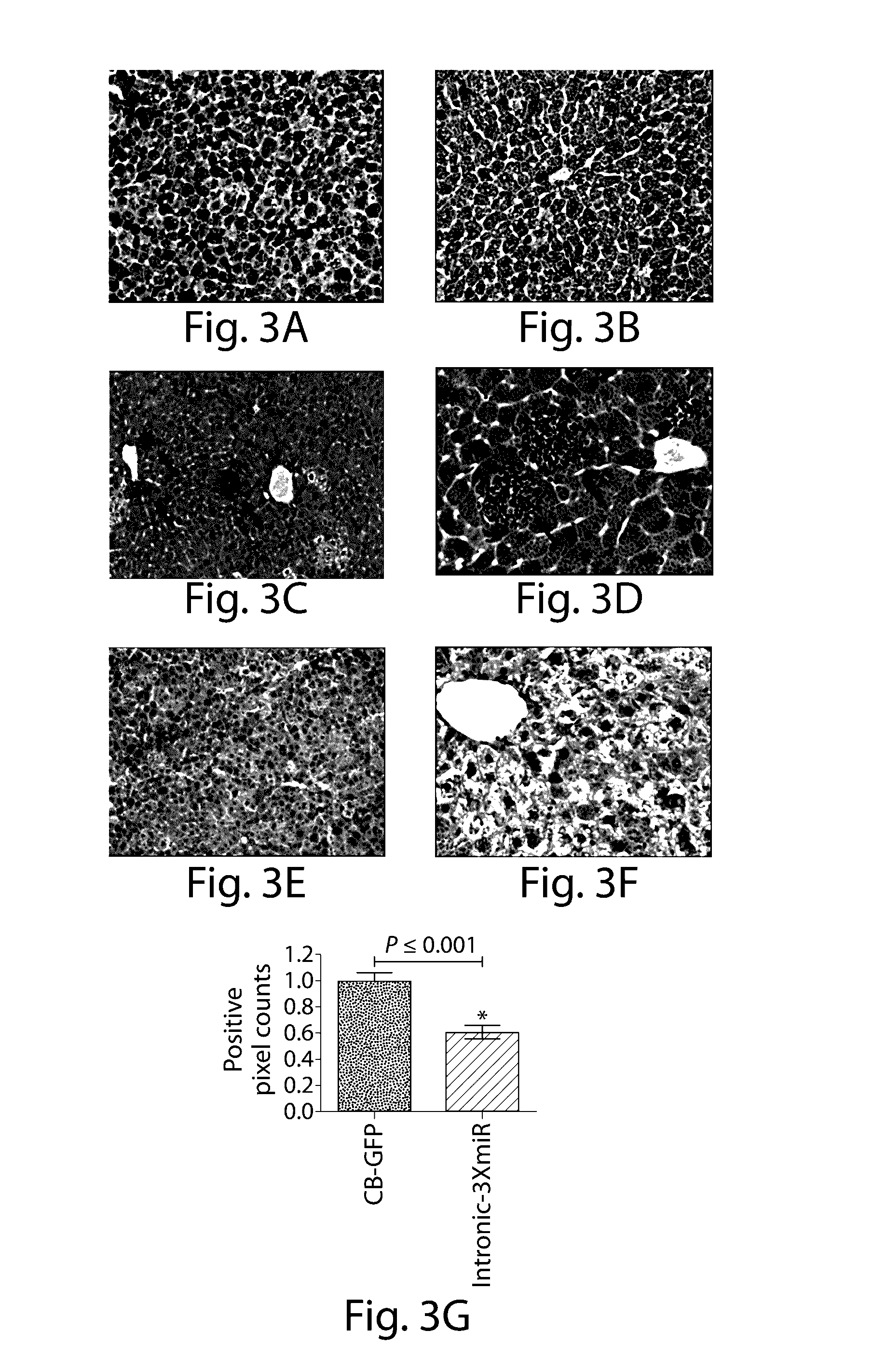
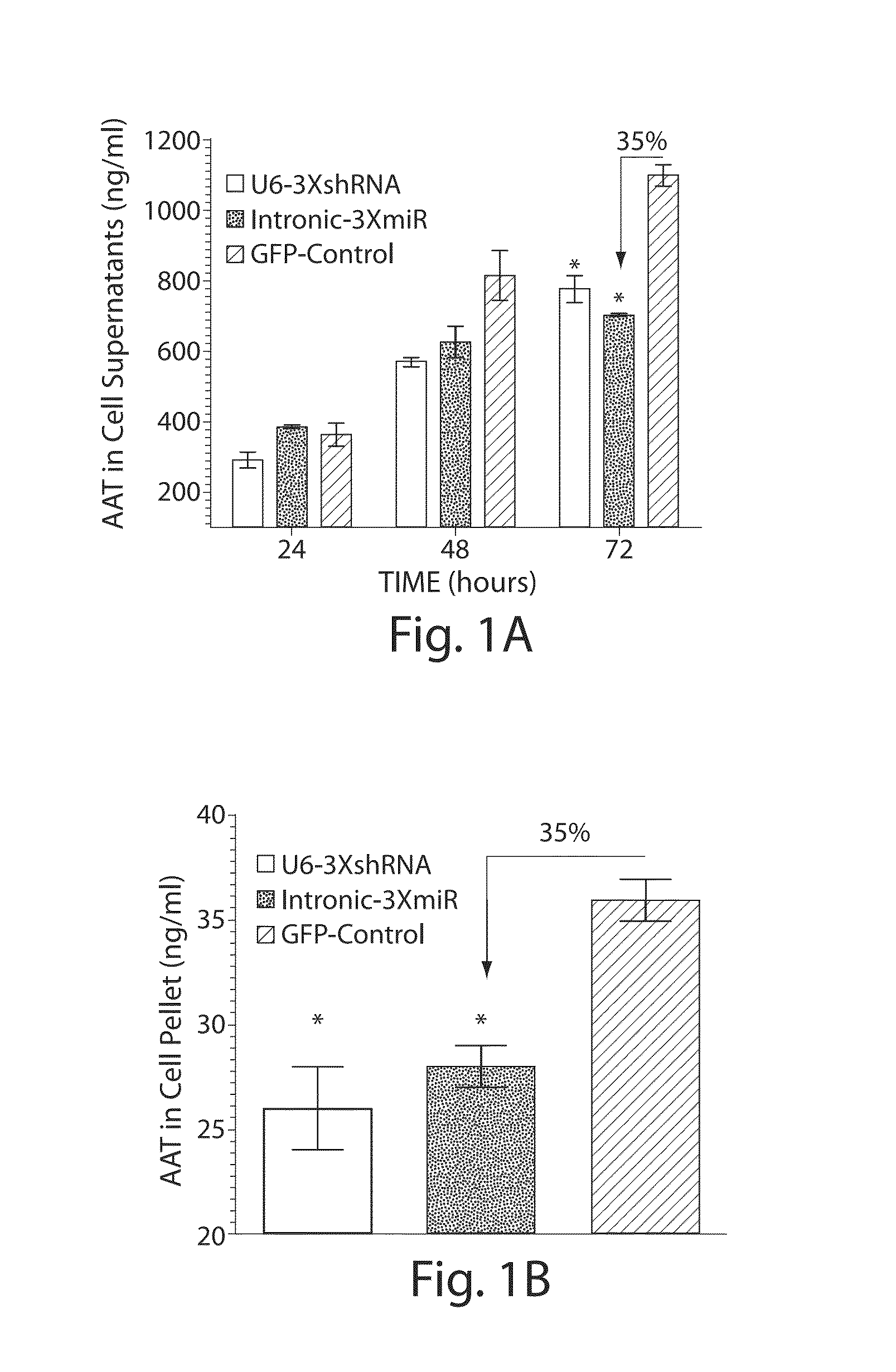
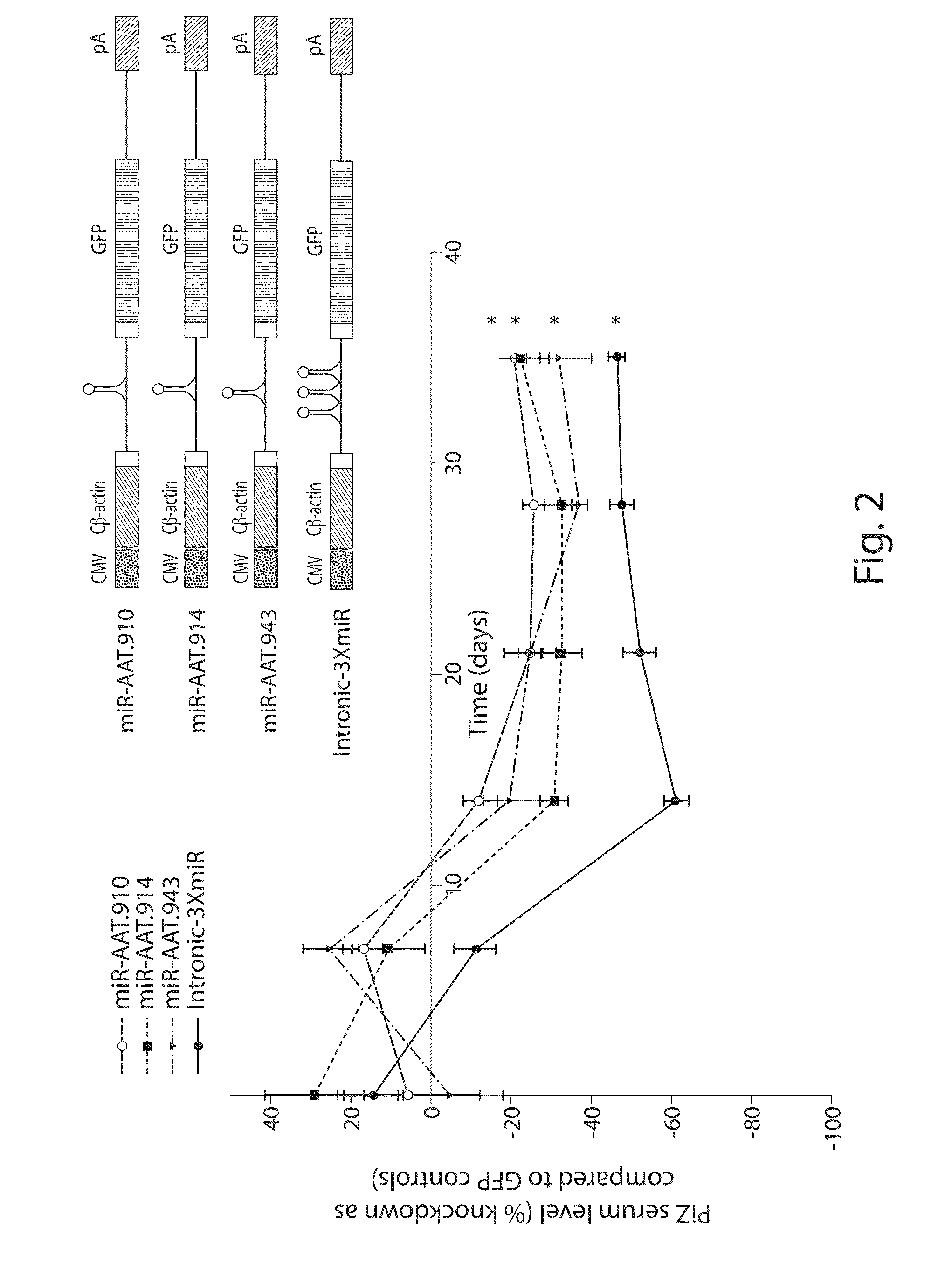
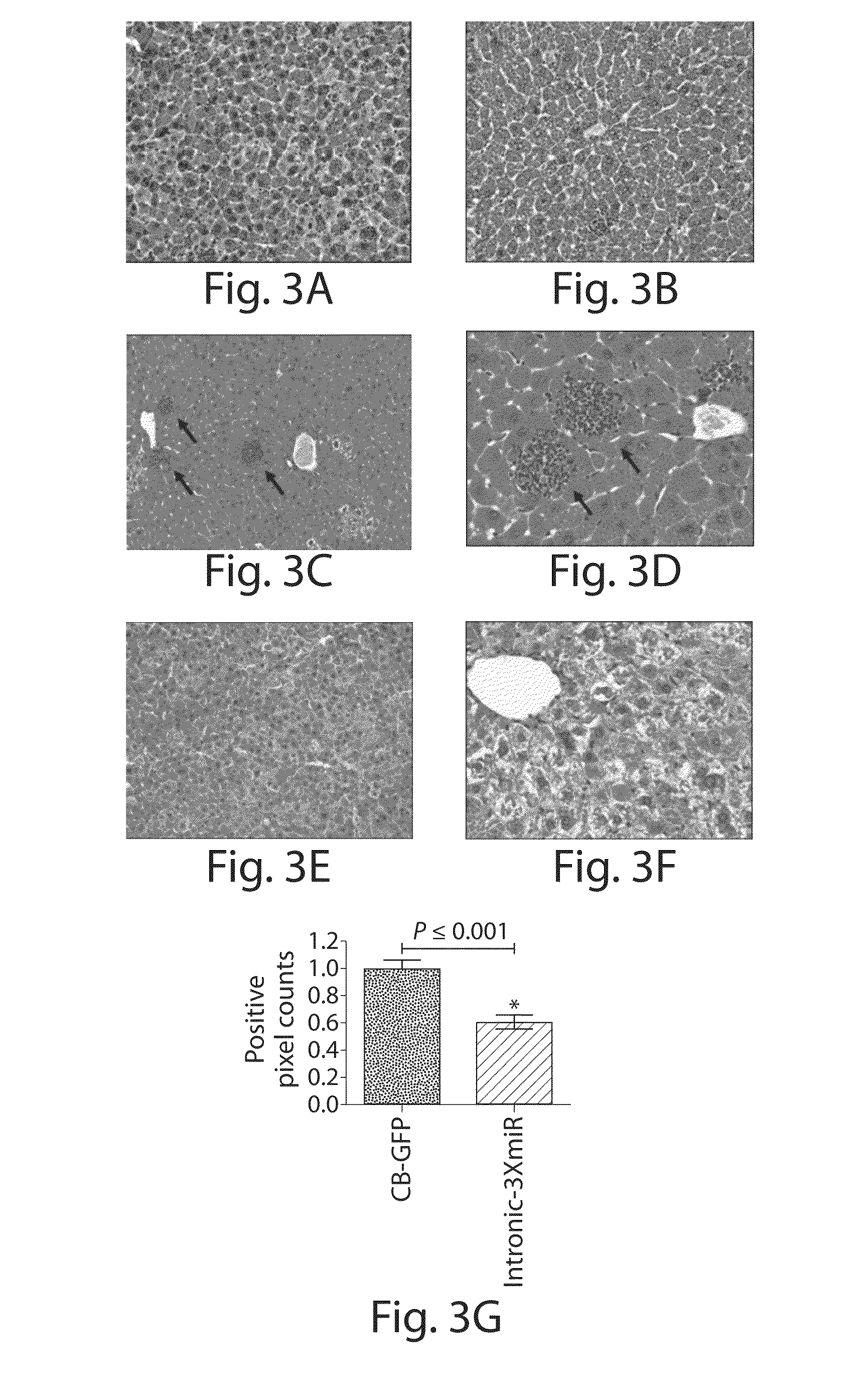
The invention relates to a RNA interference triggers for inhibiting the expression of an AAT gene through the mechanism of RNA interference. The invention also relates to a pharmaceutical composition comprising the AAT RNAi trigger together with an excipient capable of improving delivery of the RNAi trigger to a liver cell in vivo. Delivery of the AAT RNAi trigger to liver cells in vivo provides for inhibition of AAT gene expression and treatment of alpha 1-antitrypsin deficiency and associated diseases.
Owner:ARROWHEAD PHARMA INC
Alpha-1 antitrypsin variant, preparation method thereof and use thereof
ActiveUS9051395B2Improve stabilityExtended half-lifeFactor VIIDipeptide ingredientsHalf-lifeBlood drug concentration
A novel alpha-1 antitrypsin variant, a method of preparing the same, and use thereof are provided. The alpha-1 antitrypsin variant has excellent stability in the body and maintains an inhibitory effect on elastase activities because the blood half-life (t1 / 2) and the area under blood drug concentration vs. time curve (AUC) are remarkably increased by adding an N-glycosylation site in animal cells through amino acid mutation between 1st and 25th positions of the N-terminus of alpha-1 antitrypsin. Therefore, the alpha-1 antitrypsin variant can be useful in preventing or treating alpha-1 antitrypsin deficiency.
Owner:ALTEOGEN
Classification and diagnosis of the molecular basis of cholestasis
InactiveUS20080254994A1Sugar derivativesMicrobiological testing/measurementNucleotideNucleotide sequencing
The methods and compositions of the invention find use in the clinical diagnosis of cholestasis related syndromes, particularly PFIC types 1, 2, and 3; BRIC types 1 and 2; Alagille syndrome, and alpha 1-antitrypsin deficiency. The compositions of the invention include isolated nucleic acid molecules and oligonucleotide pairs suitable for use in amplifying regions of cholestasis related genes. Compositions of the invention include a cholestasis related gene resequencing microarray suitable for determining the nucleotide sequence of a region of a cholestasis related gene. Knowledge of the nucleotide sequence of one or more regions of a patient's cholestasis related gene allows diagnosis of the patient's syndrome.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Novel Genes, Compositions, and Methods for Modulating the Unfolded Protein Response
InactiveUS20110207801A1Increased transactivation potentialIncrease volumeOrganic active ingredientsSugar derivativesDiseaseAutoimmune disease
The present invention relates to methods and compositions for modulating the unfolded protein response. The method further relates to methods and compositions for the treatment and diagnosis of protein conformational diseases or disorders, including, but not limited to, α1-antitrypsin deficiency, cystic fibrosis, and autoimmune diseases and disorders. The invention further provides methods for modulating the unfolded protein response by modulating XBP1 mRNA splicing.
Owner:RGT UNIV OF MICHIGAN
Neutrophil inflammation inhibitor and uses thereof
ActiveUS10654848B1Ameliorate mitigate prevent symptomTreatment and of subjectOrganic chemistryRespiratory disorderArthritisObstructive chronic bronchitis
Disclosed herein are compounds of formula (I), and pharmaceutical compositions comprising the same. The compounds of formula (I) are neutrophilic inflammation inhibitors, thus, they are useful for treatment and / or prophylaxis of inflammatory diseases and / or disorders associated with abnormal activation of neutrophils, such as ARDS, ALI, COPD, lung fibrosis, chronic bronchitis, pulmonary emphysema, α-1 anti-trypsin deficiency, cystic fibrosis, idiopathic pulmonary fibrosis, liver injury, steatohepatitis, liver fibrosis, damages caused by ischemia and reperfusion, myocardial infarction, shock, stroke, and organ transplantation, ulcerative cholitis, vasculitis, SLE, sepsis, SIRS, arthritis, psoriasis, atopic dermatitis, and inflammatory skin diseases.
Owner:CHANG GUNG UNIVERSITY OF SCIENCE AND TECHNOLOGY +2
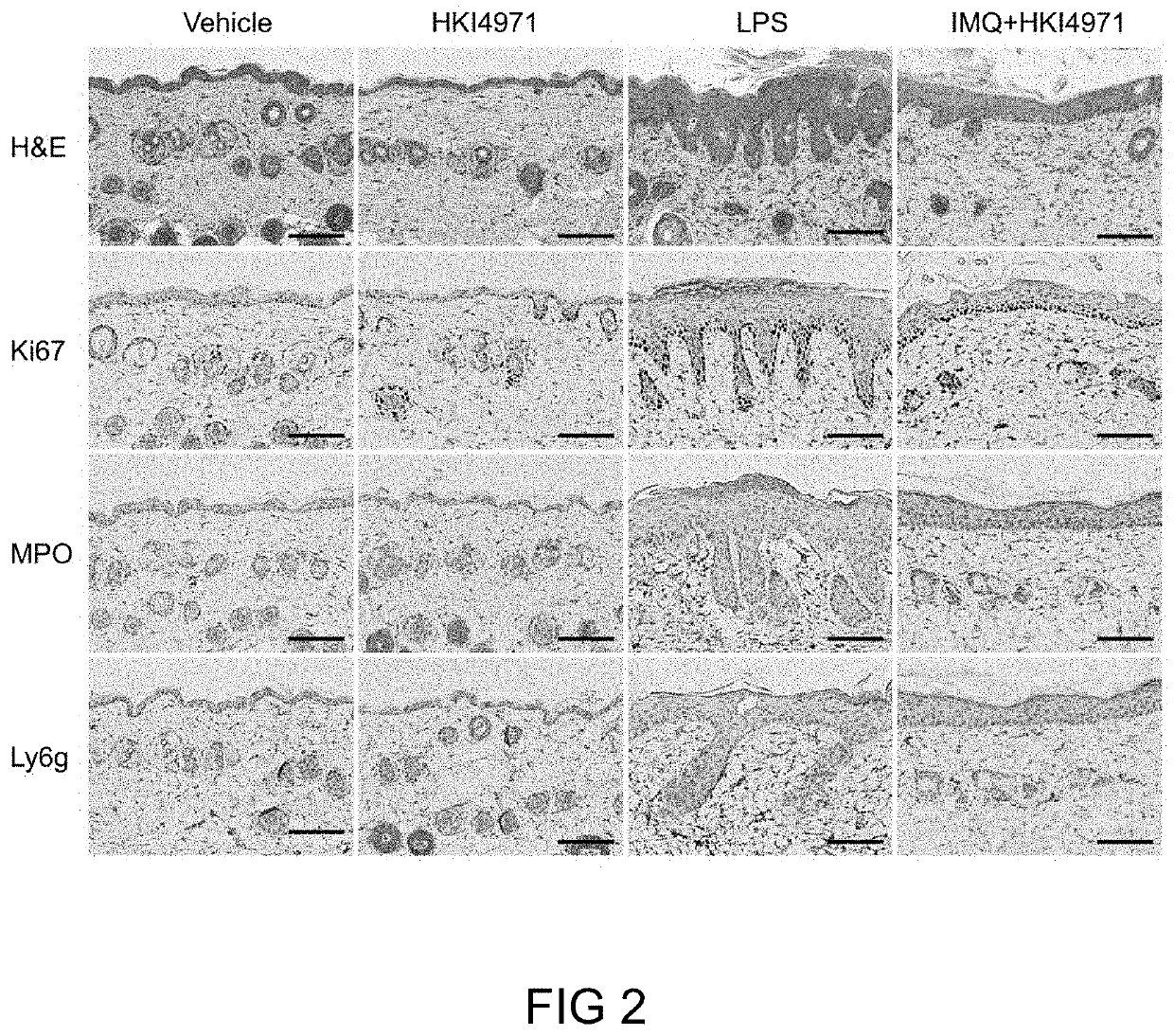
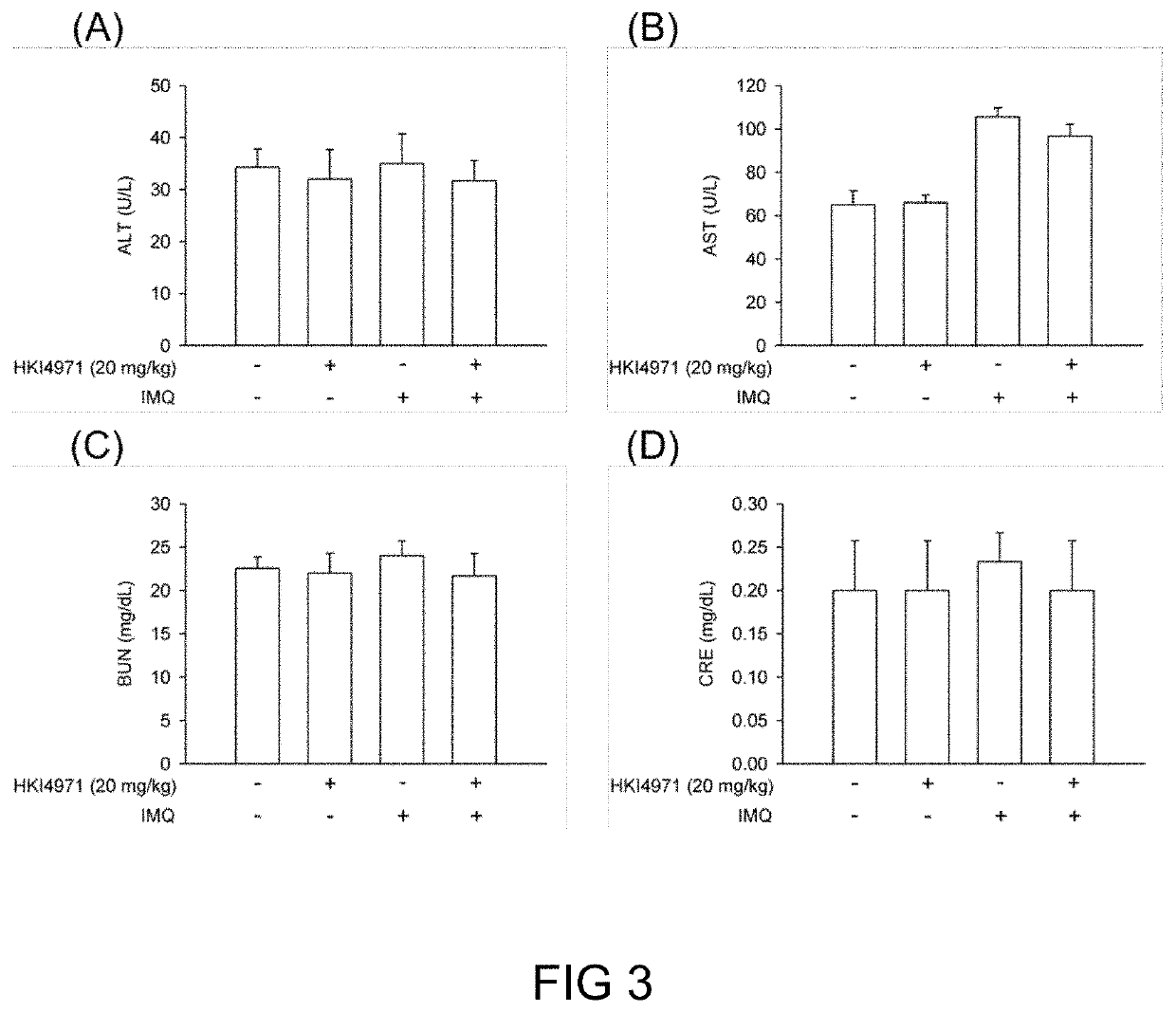
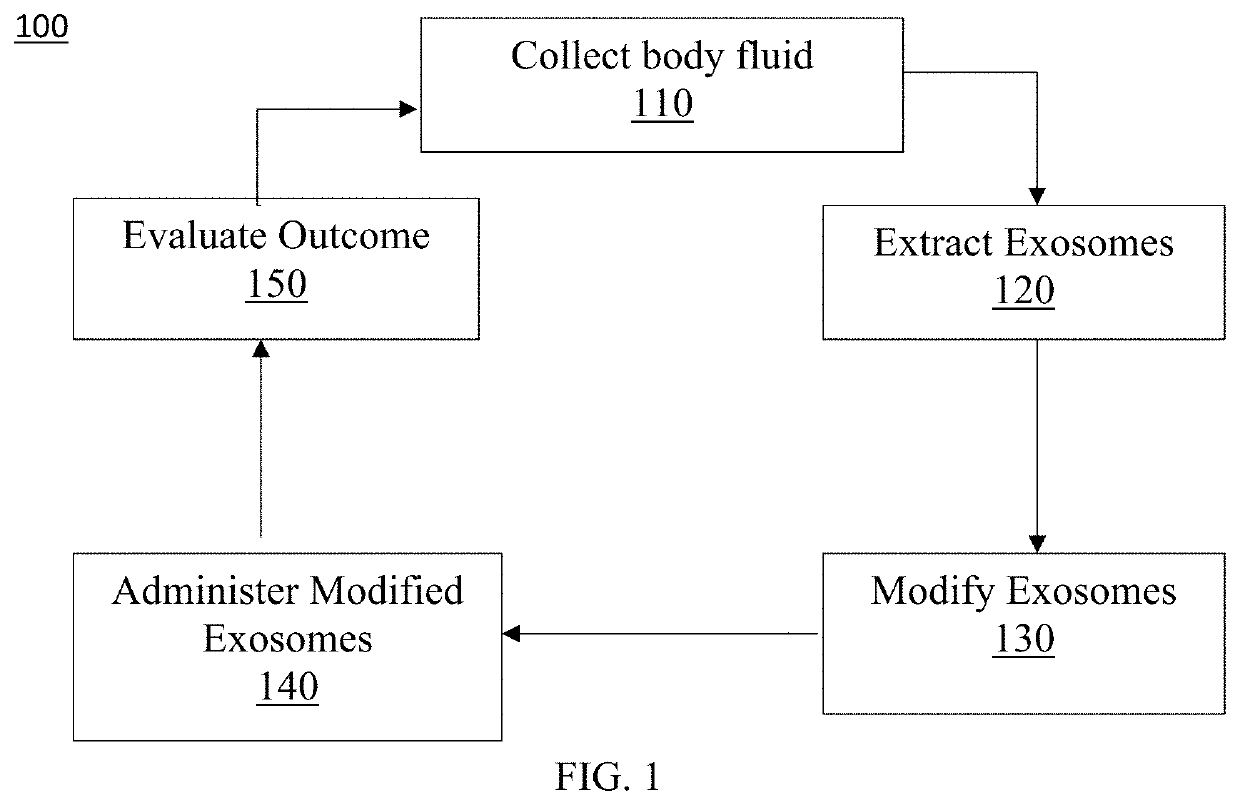
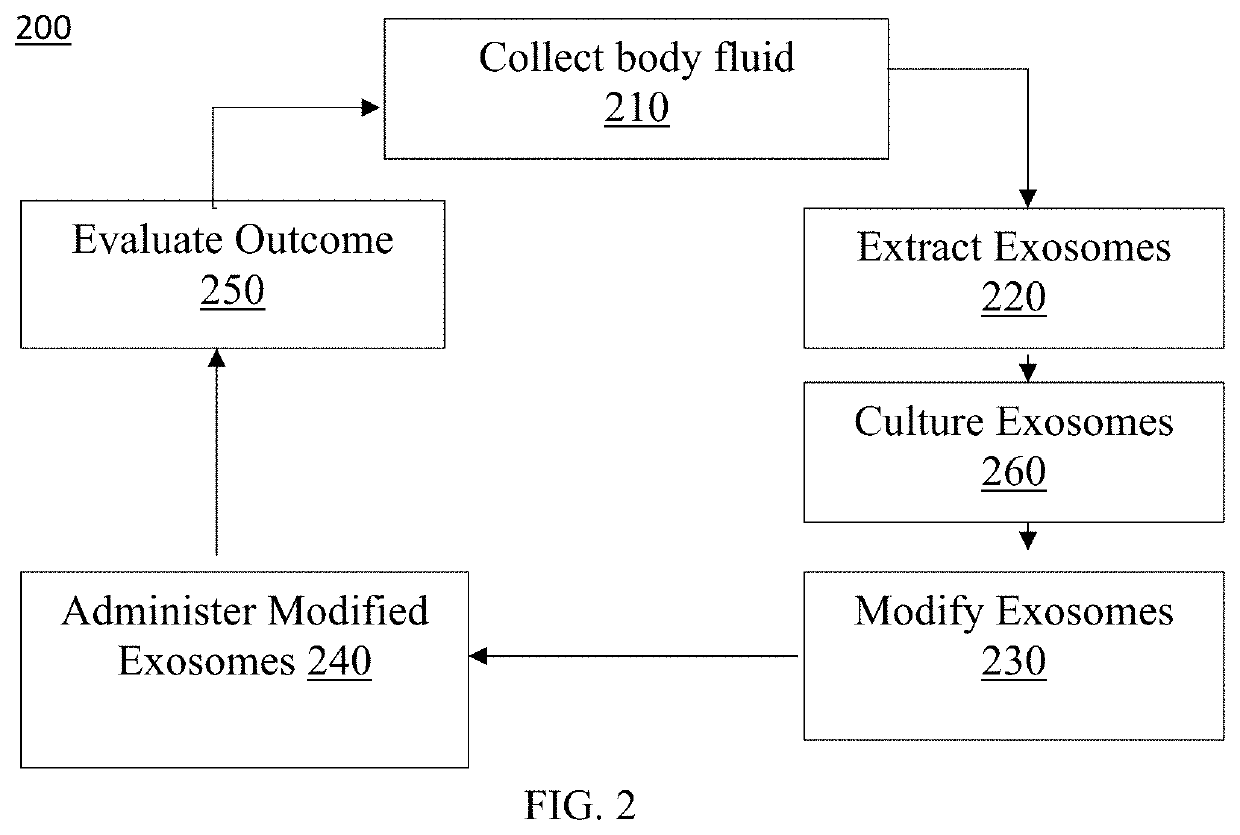
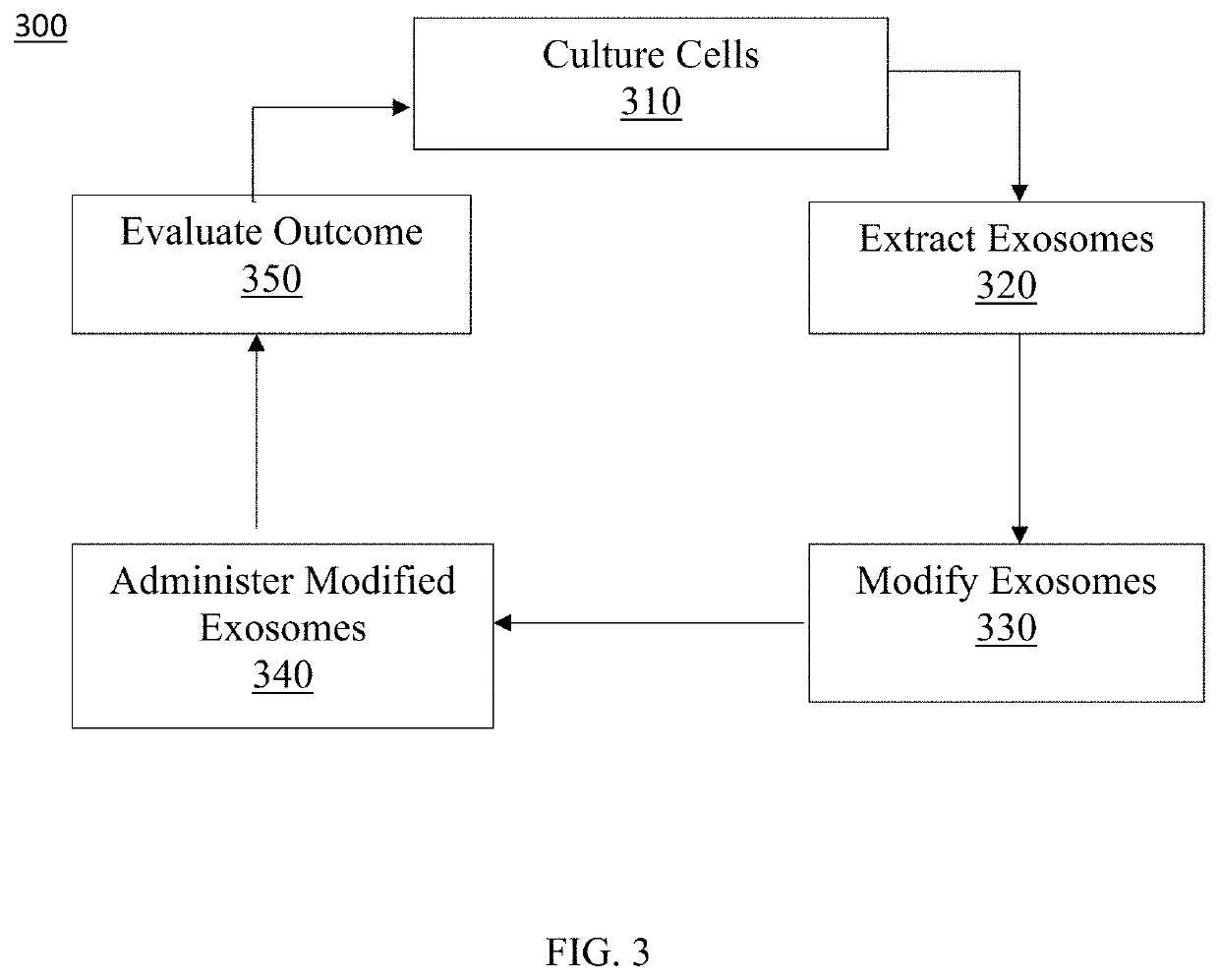
Exosome loaded therapeutics for the treatment of non-alcoholic steatohepatitis, diabetes mellitus type 1 and type 2, atherosclerotic cardiovascular disease, and alpha 1 antitrypsin deficiency
PendingUS20200157541A1Superior in potency and efficacyHigh potencySpecial deliveryMicroencapsulation basedCytoplasmTrypsin
A composition for delivering a cargo to the cytoplasm of a cell, wherein the cargo treats Non-Alcoholic Steatohepatitis, Non-Alcoholic Fatty Liver Disease, Diabetes Mellitus Type 1 and Type 2, Atherosclerotic Cardiovascular Disease, and Alpha 1 Antitrypsin Deficiency. In another embodiment, the composition comprises: an exosome; cargo located inside the exosome, wherein the cargo comprises short interference RNA (siRNA) that depletes sodium-glucose linked transporter active sites. In another embodiment, the composition comprises: an exosome; cargo location inside the exosome, wherein the cargo corrects the missense SERPINA1 mutation from ‘Z’ to ‘M’.
Owner:EXOSOME THERAPEUTICS INC
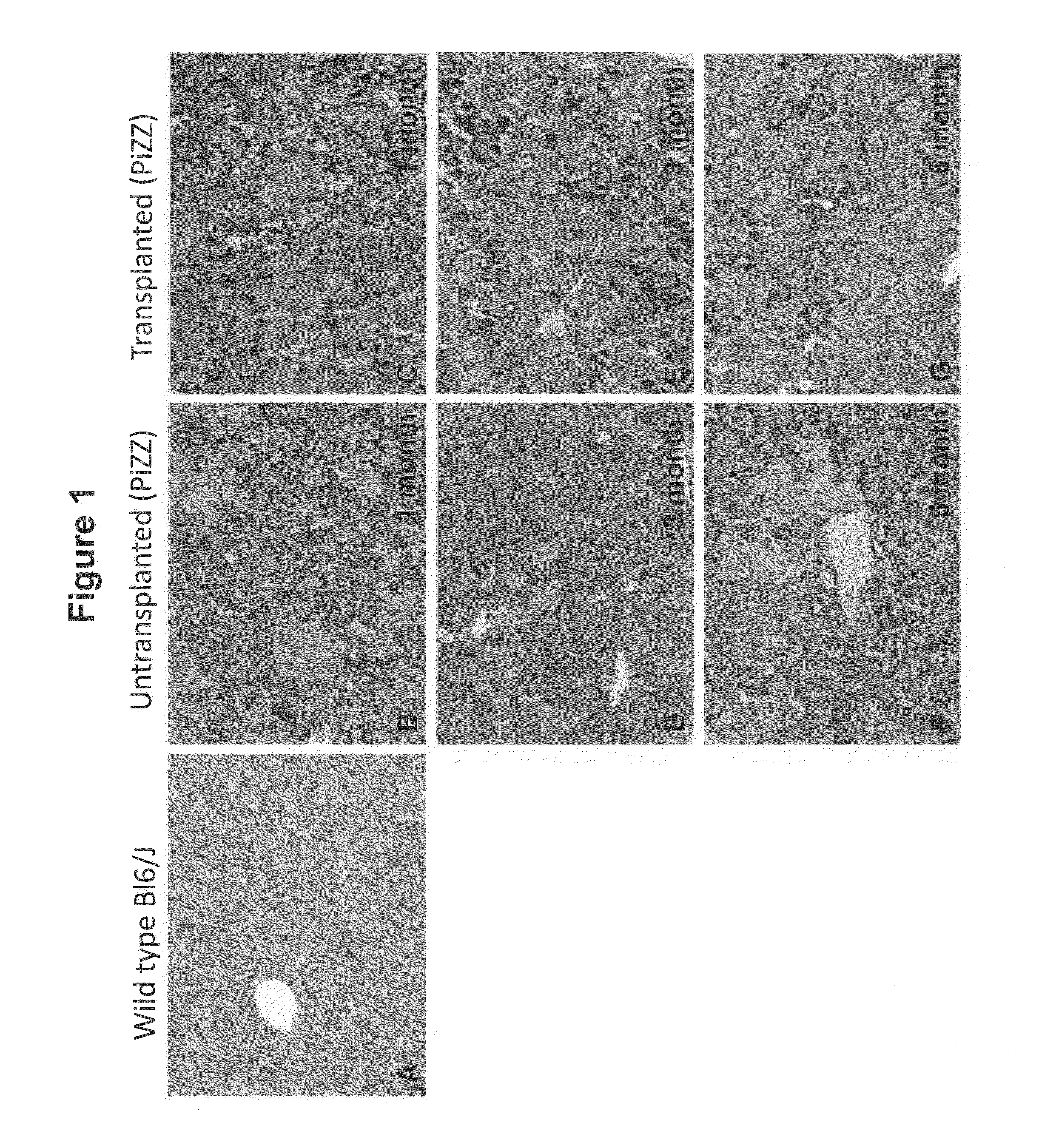
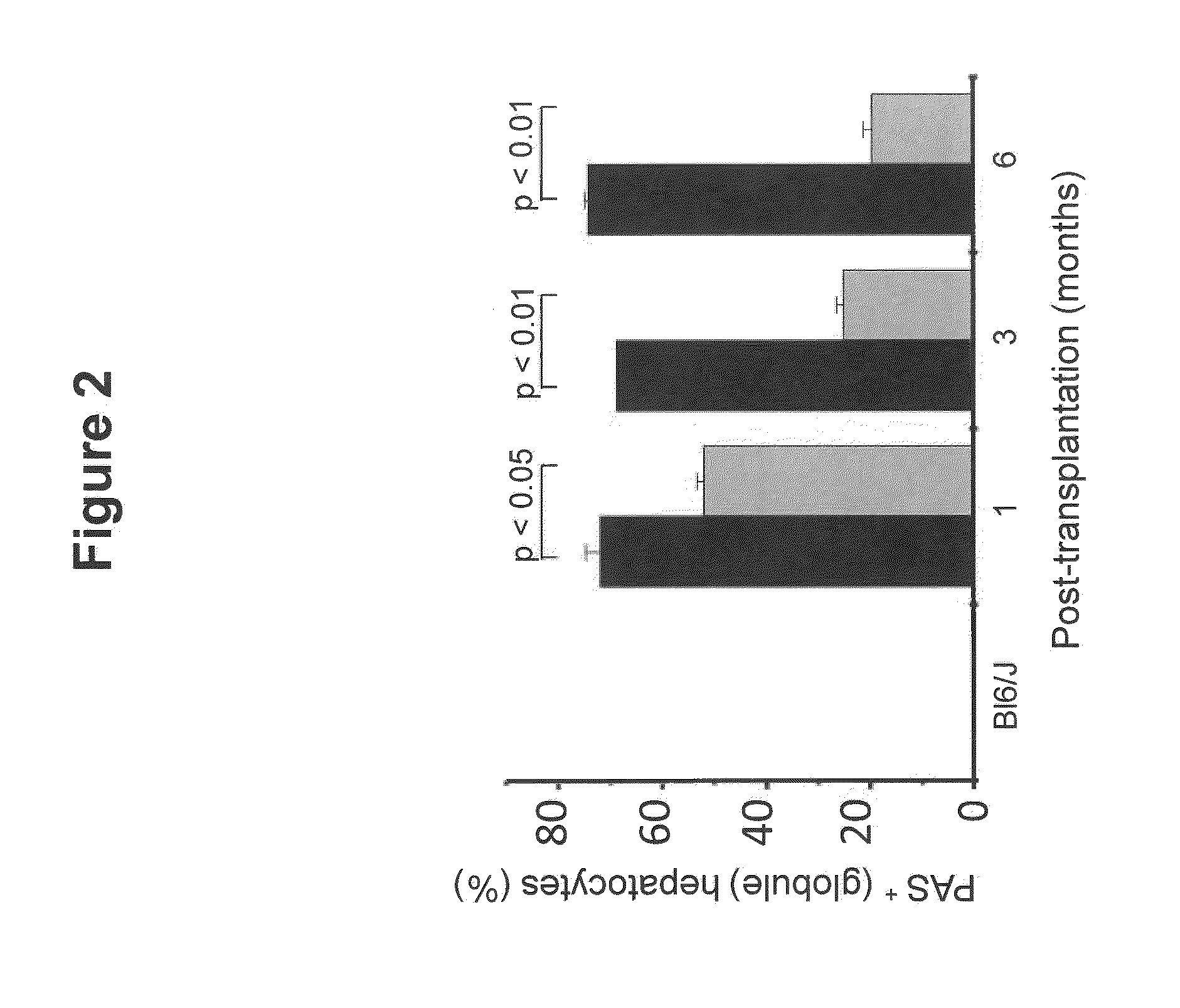
Bone Marrow-Derived Cells Ameliorates The Pathological Consequences Of The Liver In Case Of Alpha1-Antitrypsin Deficiency
The present invention is based on the findings that bone marrow (BM)-derived progenitor cells more specifically mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs) and uncommitted hematopoietic cells (lin−) are capable of regenerating liver in case of injury. The invention provides a method for treating genetic disorder like Alpha1-antitrypsin deficiency (A1-ATD) by administering BM derived Lin− cells in human mutant A1-AT expressing transgenic mouse model. The invention also provides the state of art for replacement of mutant host hepatocytes by transplanting wild-type uncommitted donor (lin−) cells.
Owner:NATIONAL INSTUTUTE OF IMMUNOLOGY
Compositions and methods for gene delivery to the airways and/or lungs
PendingUS20210189427A1Low cytotoxicityDispersion deliveryPeptide/protein ingredientsGene deliveryDisease
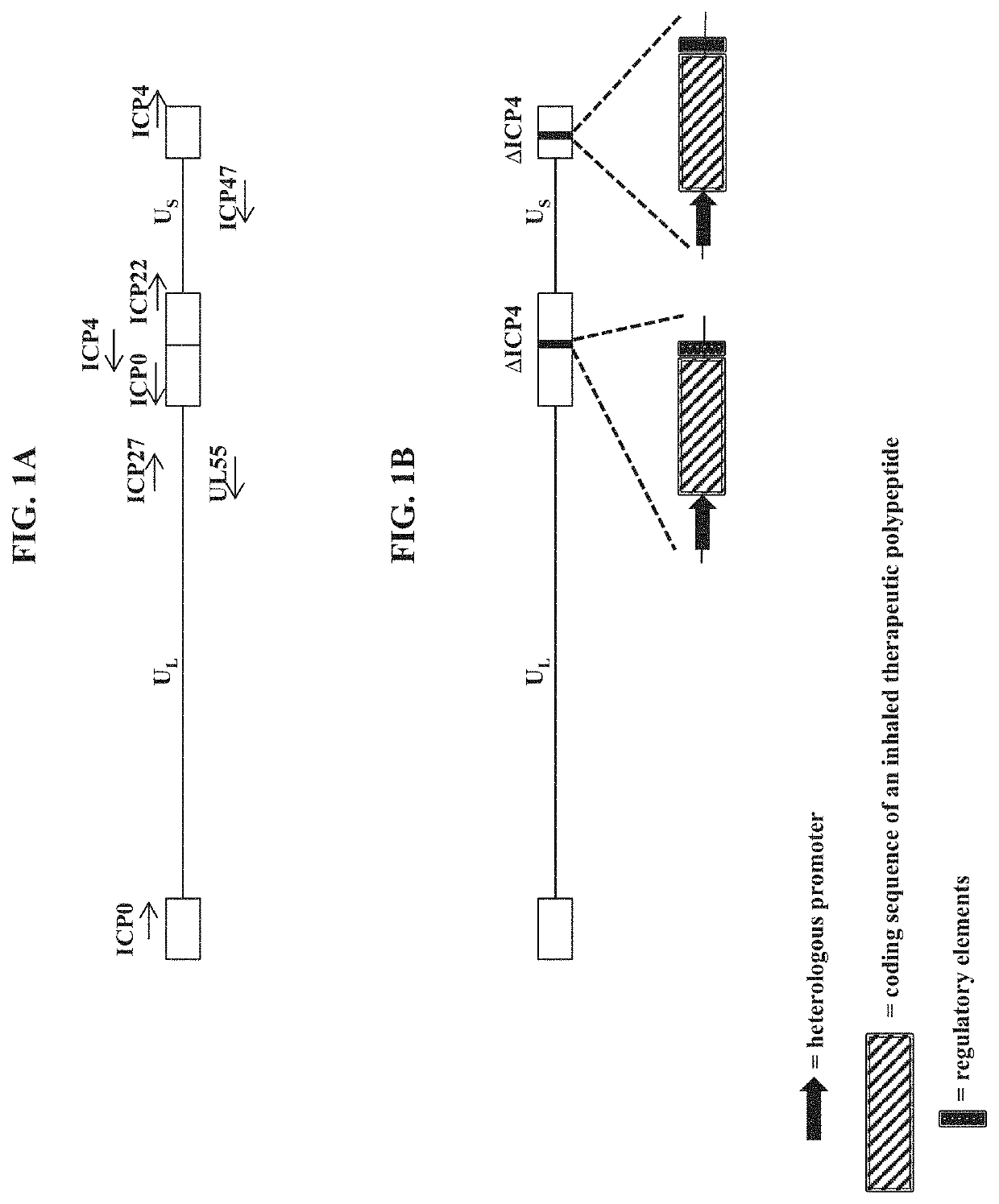
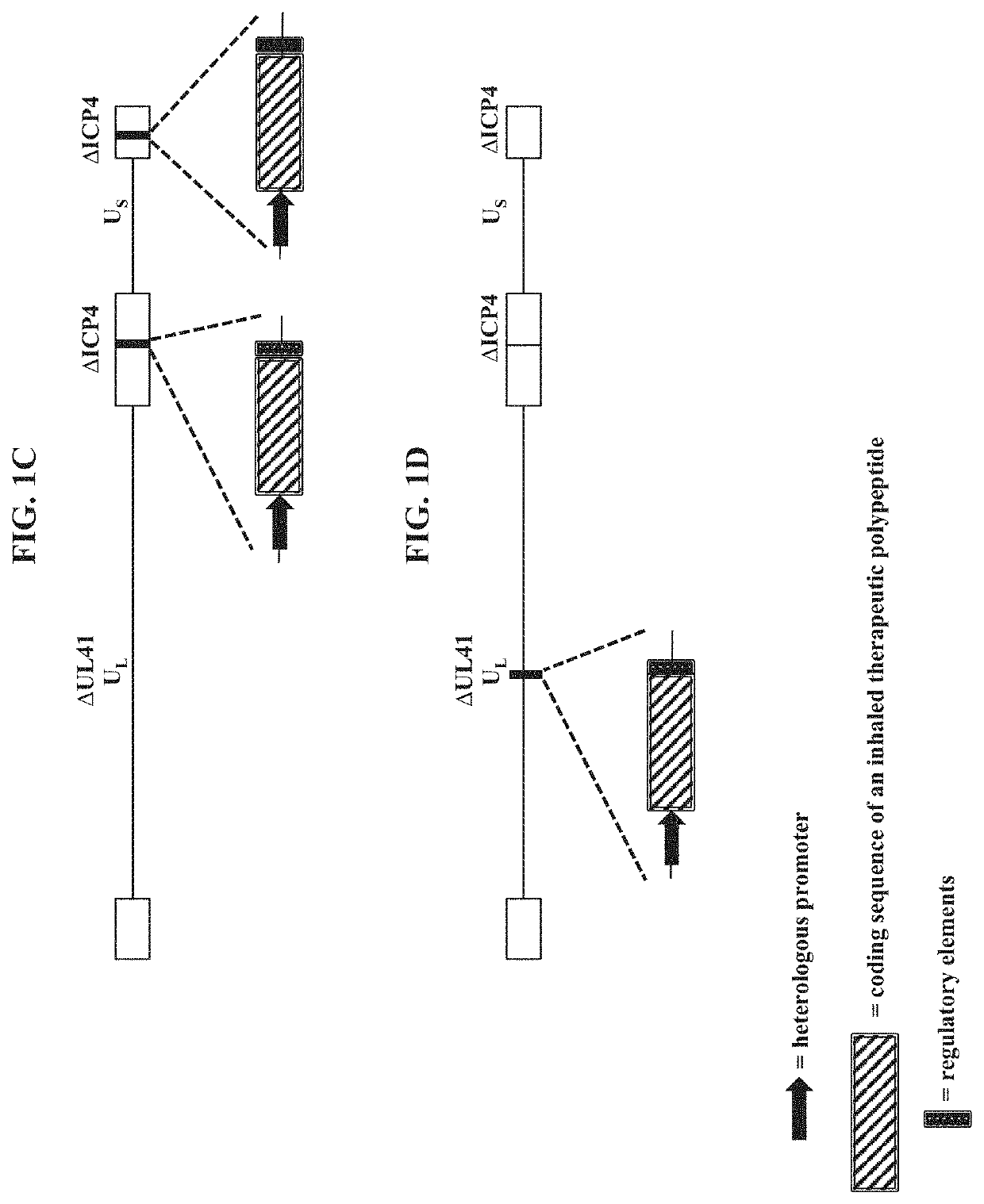
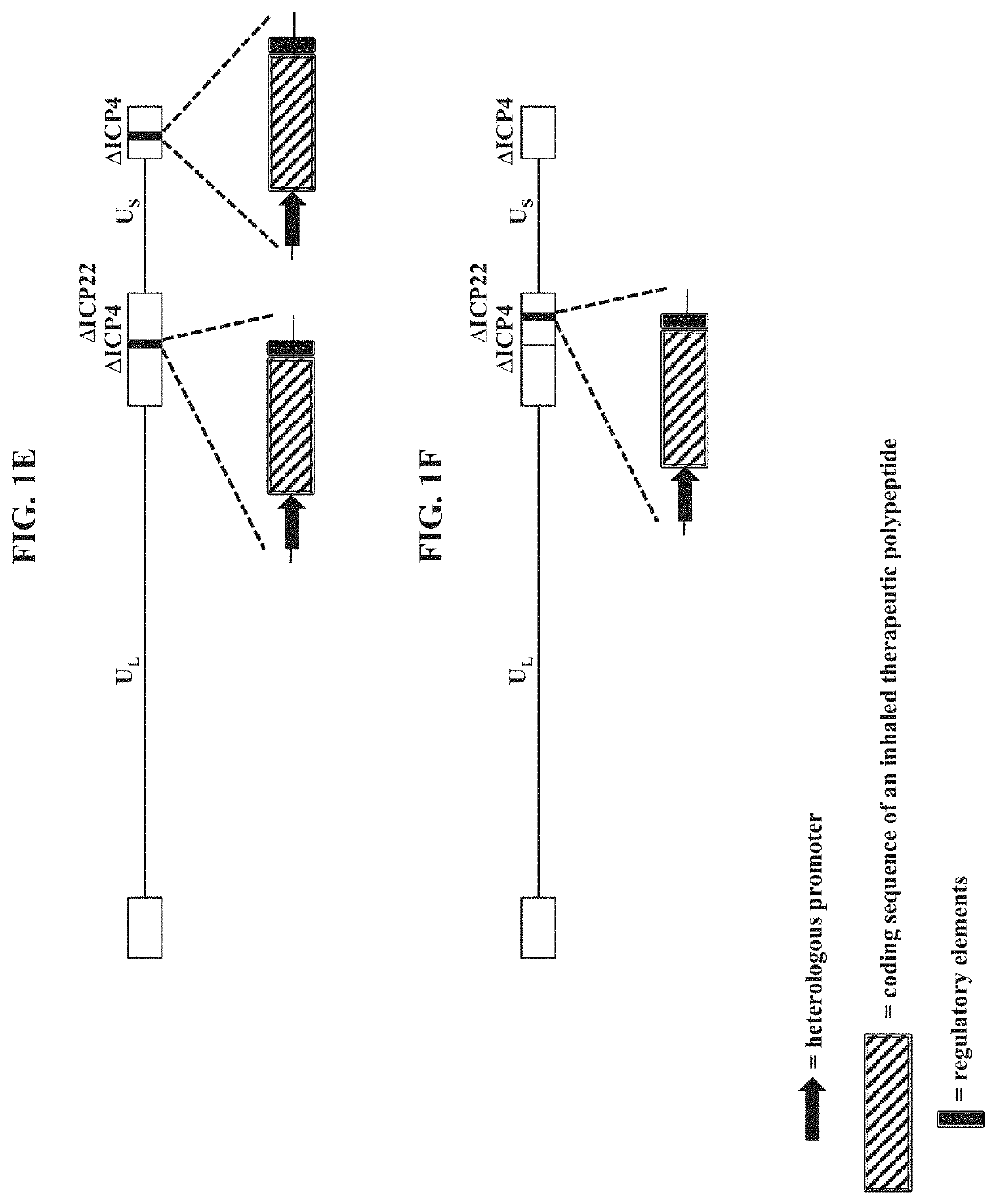
The present disclosure provides recombinant nucleic acids comprising one or more polynucleotides encoding a polypeptide (e.g., an inhaled therapeutic polypeptide, such as a human alpha-1-antitrypsin polypeptide); viruses comprising the recombinant nucleic acids; compositions and formulations comprising the recombinant nucleic acids and / or viruses; methods of their use (e.g., for delivering the polypeptide to one or more cells of the respiratory tract and / or for the treatment of a disease affecting the lungs, such as alpha-1-antitrypsin deficiency); and articles of manufacture or kits thereof.
Owner:KRYSTAL BIOTECH INC
Novel alpha-1 antitrypsin variant, preparation method thereof and use thereof
ActiveUS20140371160A1Improve stabilityMaintains inhibitory effect on elastase activityFactor VIIDipeptide ingredientsHalf-lifeBlood drug concentration
A novel alpha-1 antitrypsin variant, a method of preparing the same, and use thereof are provided. The alpha-1 antitrypsin variant has excellent stability in the body and maintains an inhibitory effect on elastase activities because the blood half-life (t1 / 2) and the area under blood drug concentration vs. time curve (AUC) are remarkably increased by adding an N-glycosylation site in animal cells through amino acid mutation between 1st and 25th positions of the N-terminus of alpha-1 antitrypsin. Therefore, the alpha-1 antitrypsin variant can be useful in preventing or treating alpha-1 antitrypsin deficiency.
Owner:ALTEOGEN
Methods Of Treatment For Alpha-1 Antitrypsin Deficiency
InactiveUS20190071670A1Reduce the burden onEnhance intracellular autophagy processOrganic active ingredientsDNA/RNA fragmentationDiseaseReticulum cell
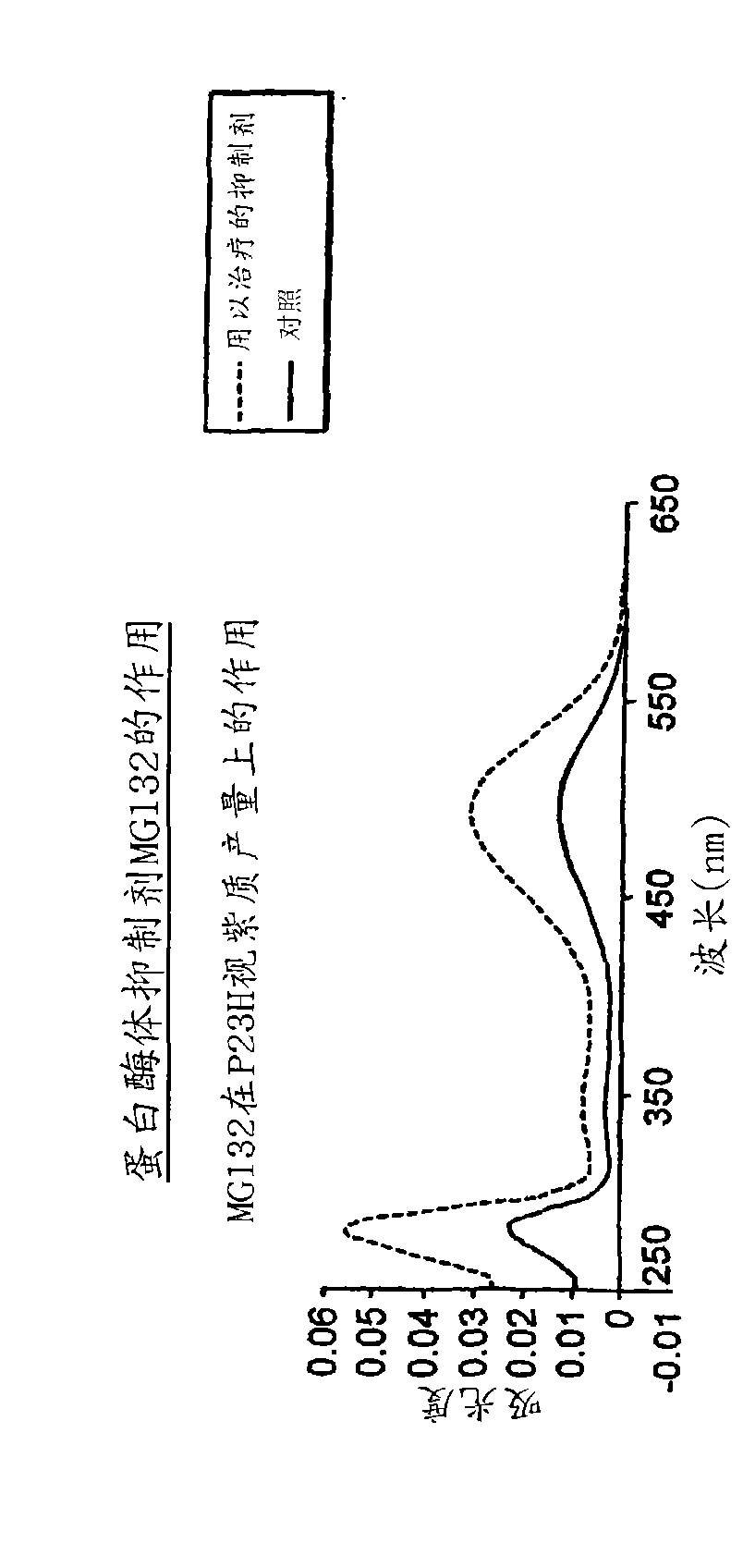
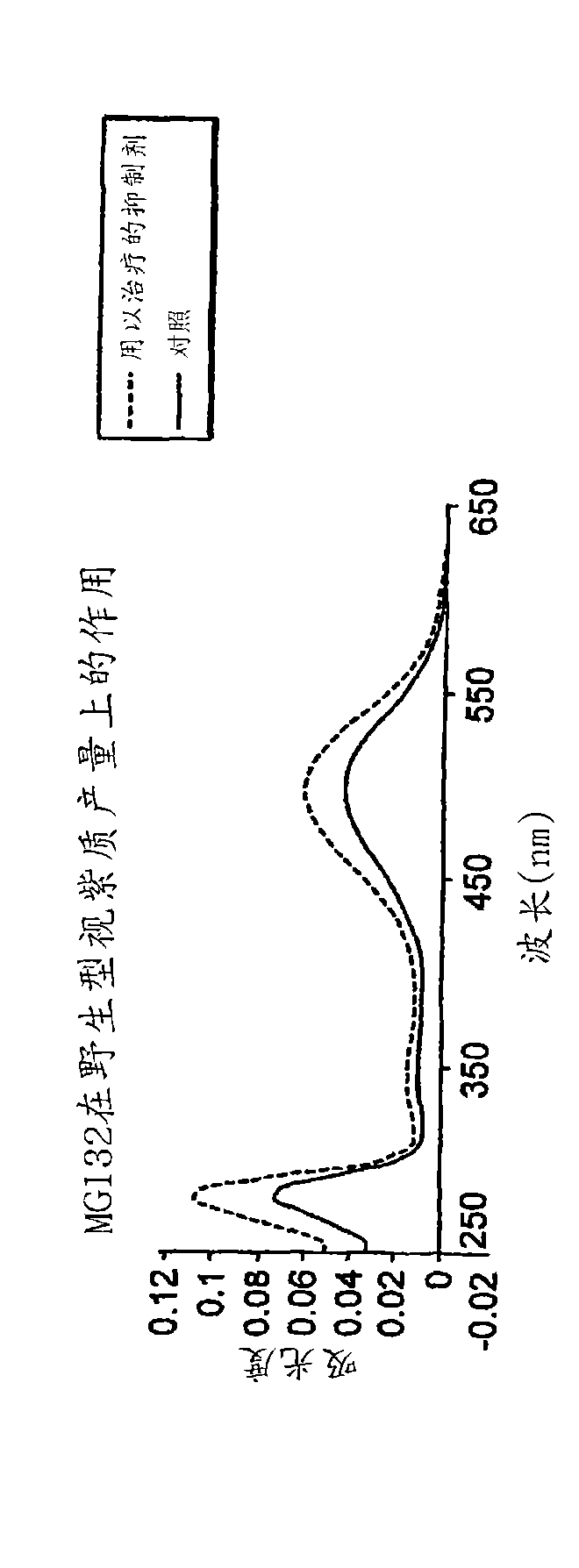
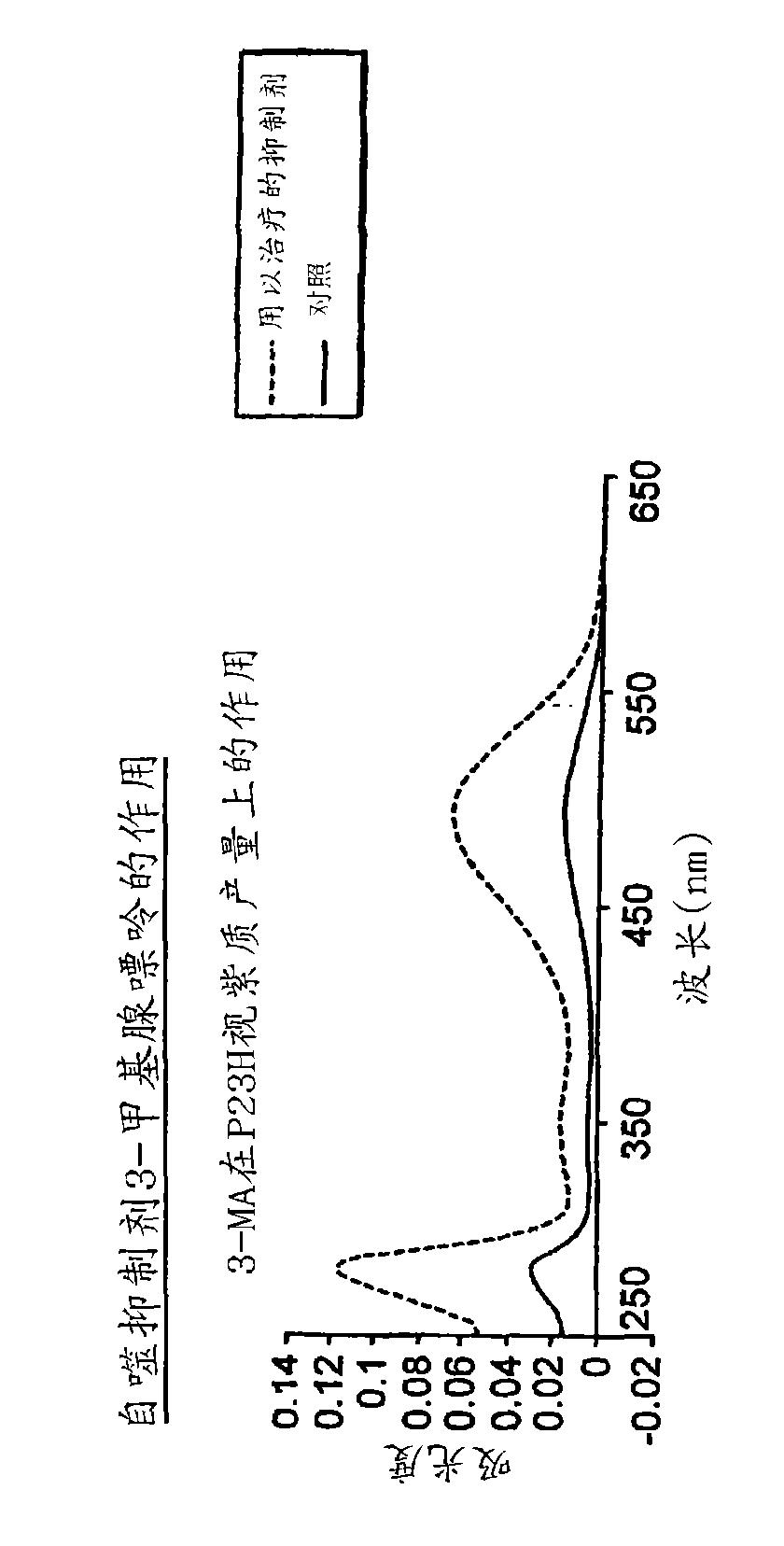
The application relates to methods of treatment and treatment regimens for Alpha-1 Antitrypsin deficiency (AATD) and the conditions, manifestations, and diseases caused by AATD, by the administration of one or more expression-inhibiting oligomeric compounds having a nucleobase sequence complementary to a coding sequence in the Alpha-1 Antitrypsin (A1AT or AAT) gene that inhibits the expression of the AAT gene, in combination with one or more autophagy enhancing agents that enhance and / or induce the endogenous autophagy mechanism to facilitate and encourage clearance of polymerized mutant AAT protein accumulated in the endoplasmic reticulum of hepatocytes. When used in combination, delivery of the expression-inhibiting oligomeric compounds to liver cells in vivo provides for inhibition of AAT gene expression and the use of autophagy enhancing agents increases the rate of the intracellular autophagy mechanism to clear polymerized mutant Z-AAT protein accumulated in cells, leading to an improved treatment of AATD and prevention and treatment of conditions and diseases associated with AATD.
Owner:ARROWHEAD PHARMA INC
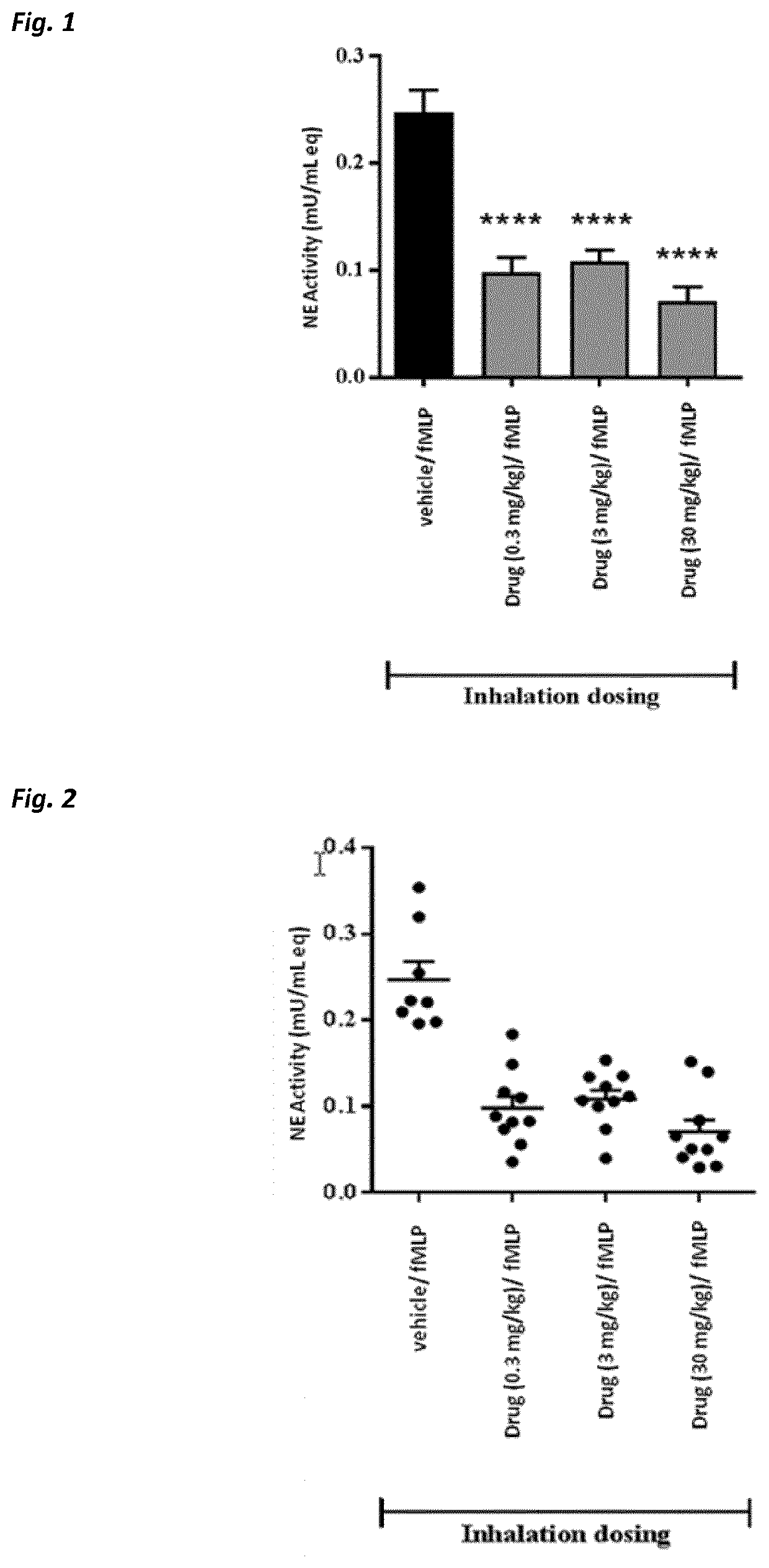
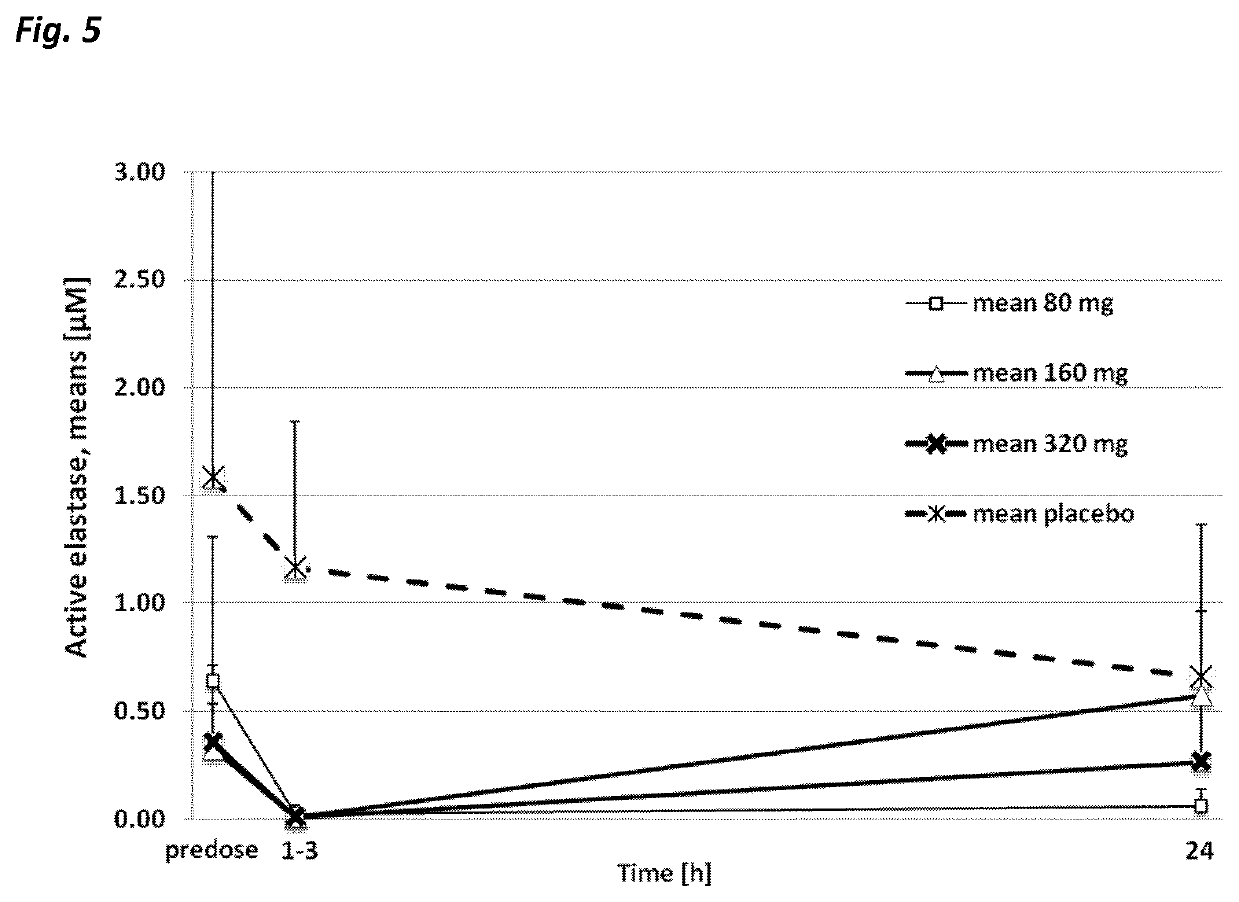
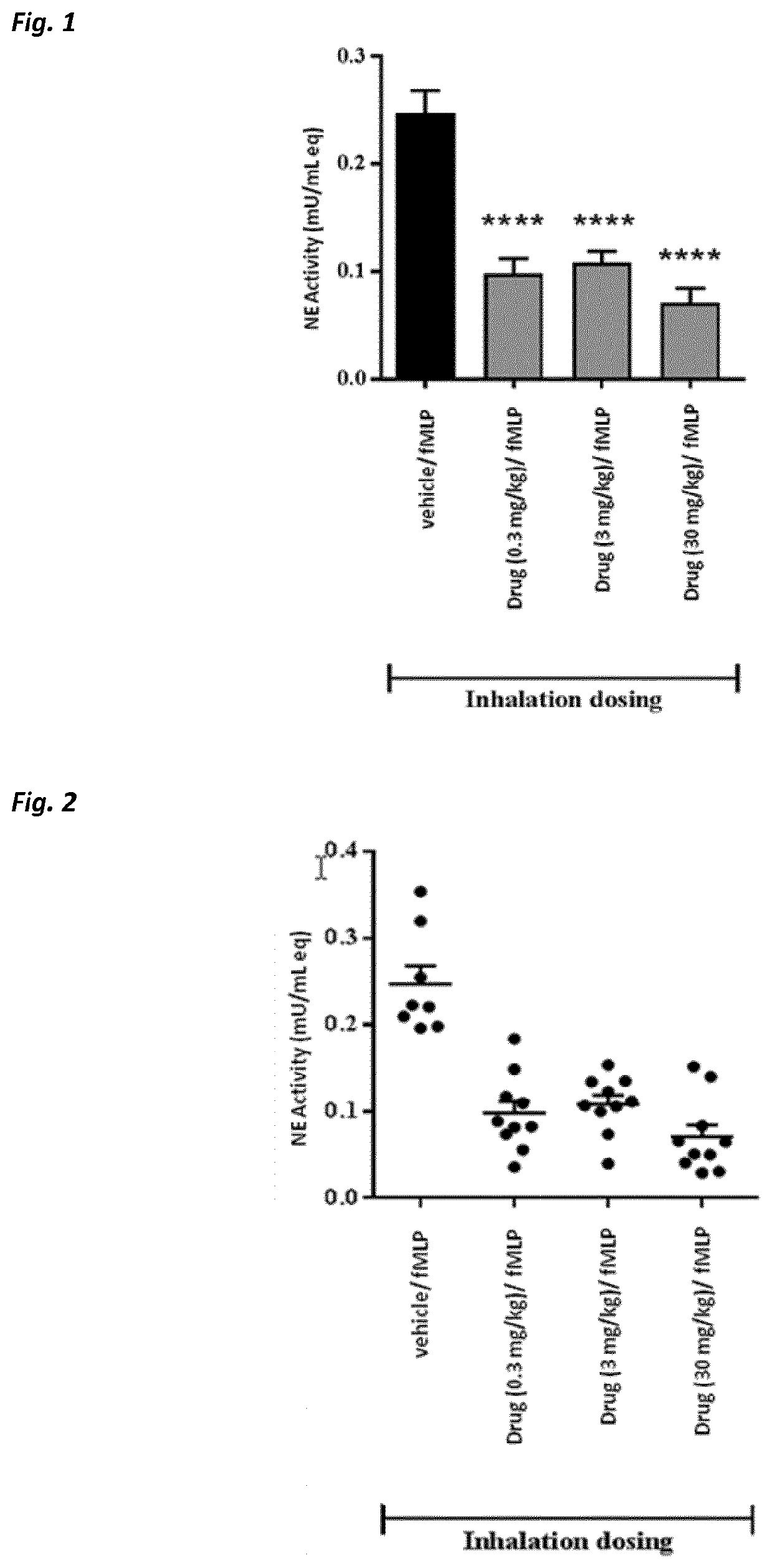
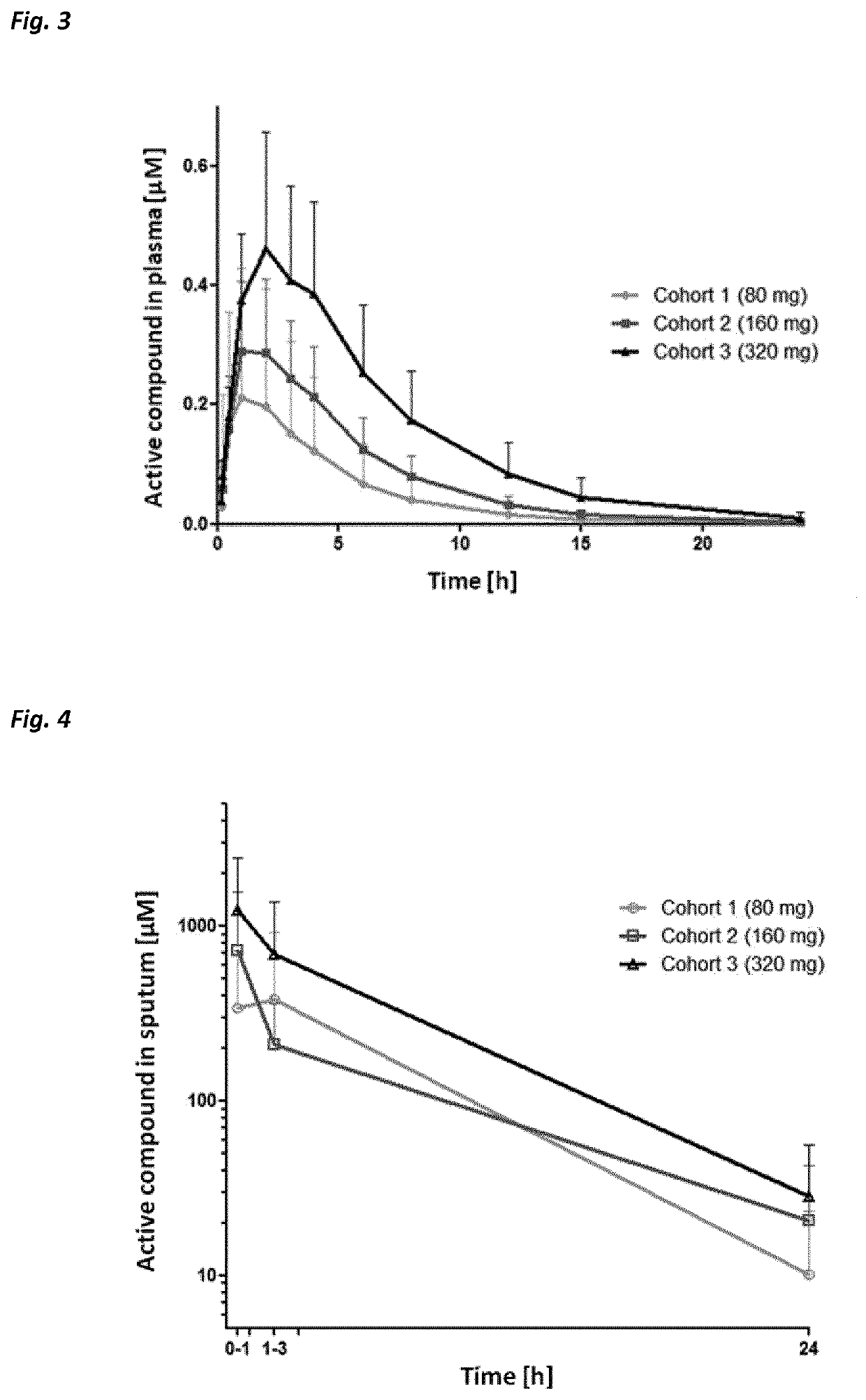
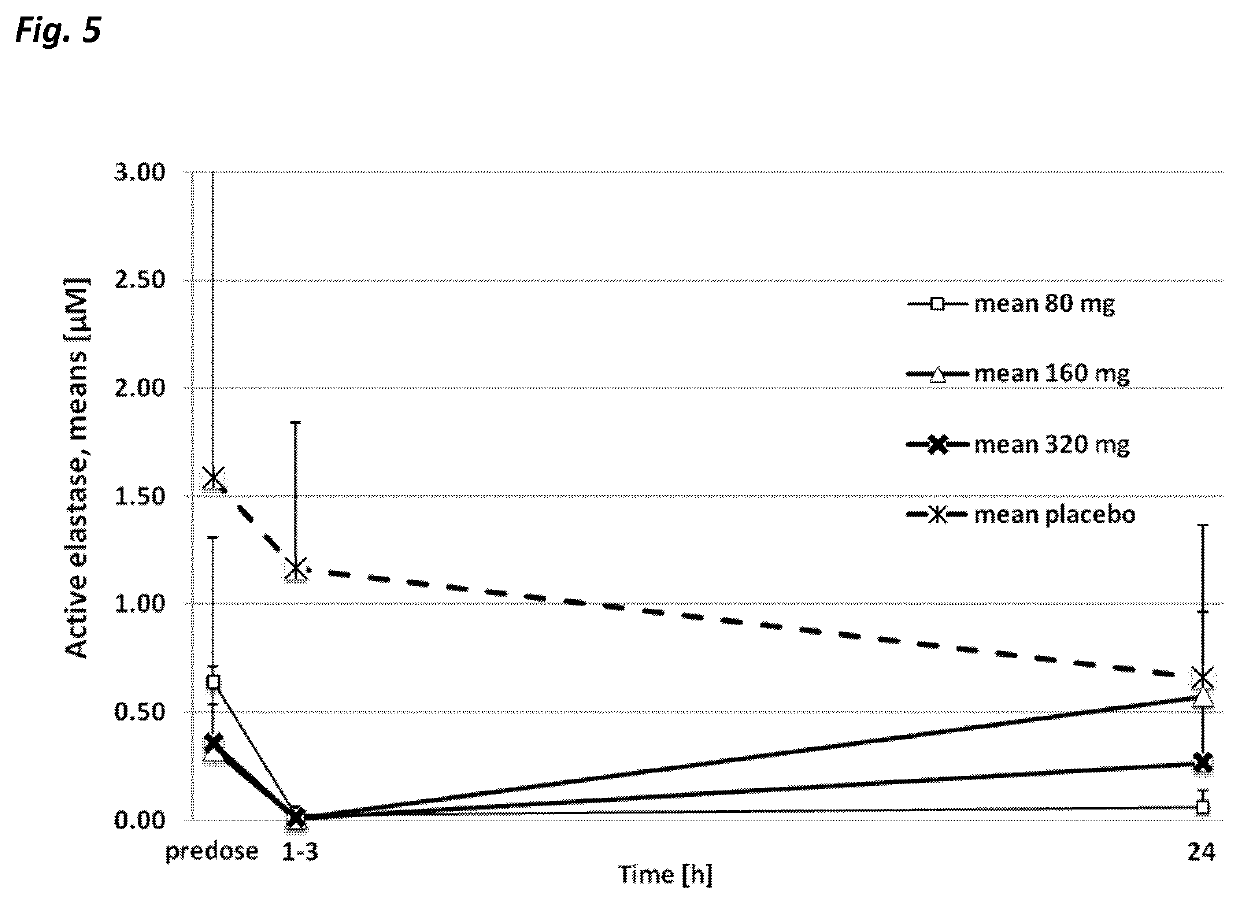
Use of neutrophil elastase inhibitors in lung diseases
The present invention relates to a method for treating chronic lung disease, in particular alpha-1 antitrypsin deficiency or emphysema caused by alpha-1 antitrypsin deficiency, with a neutrophil elastase inhibitor. The invention also relates to a pharmaceutical composition comprising a neutrophil elastase inhibitor.
Owner:PH PHARMA CO LTD
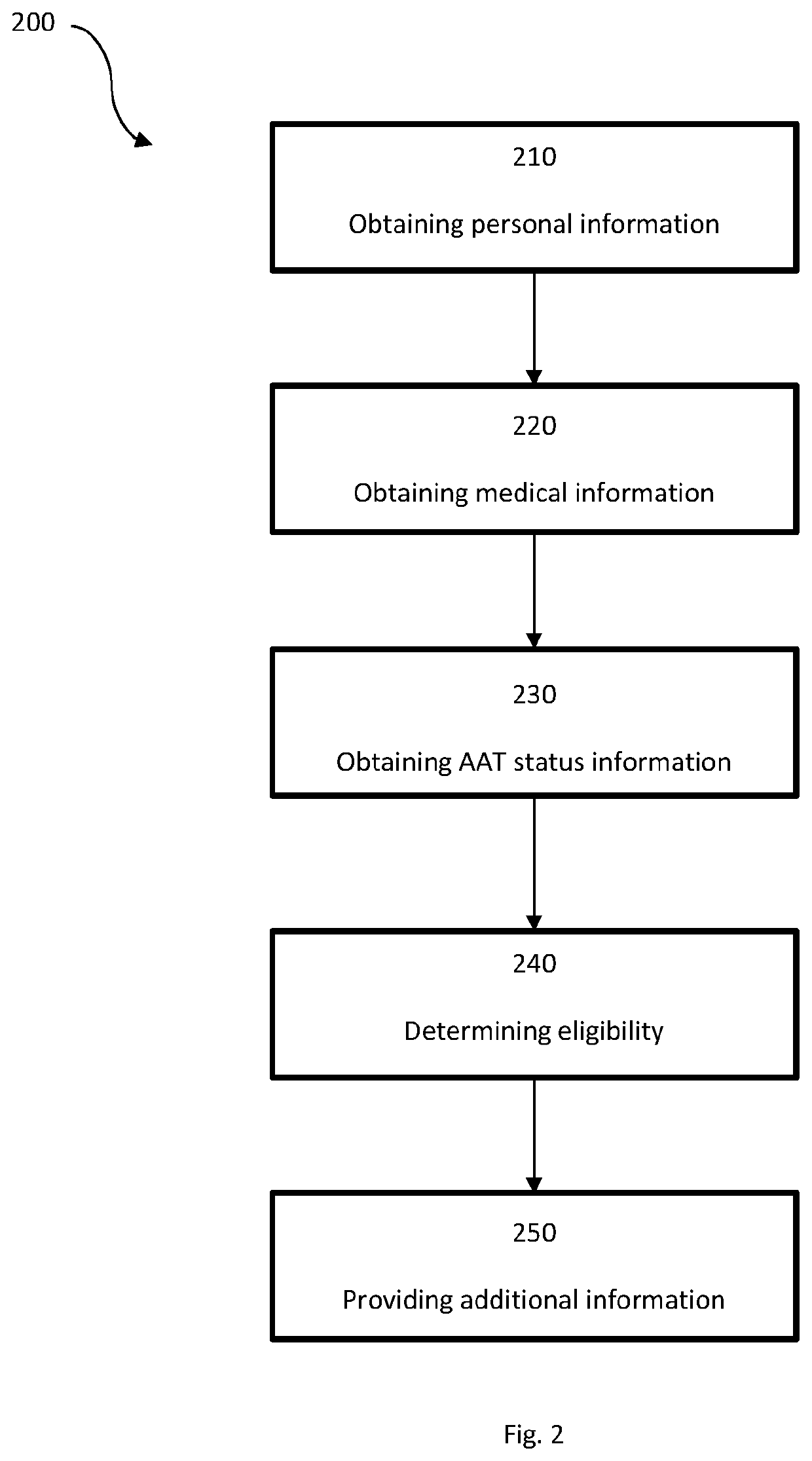
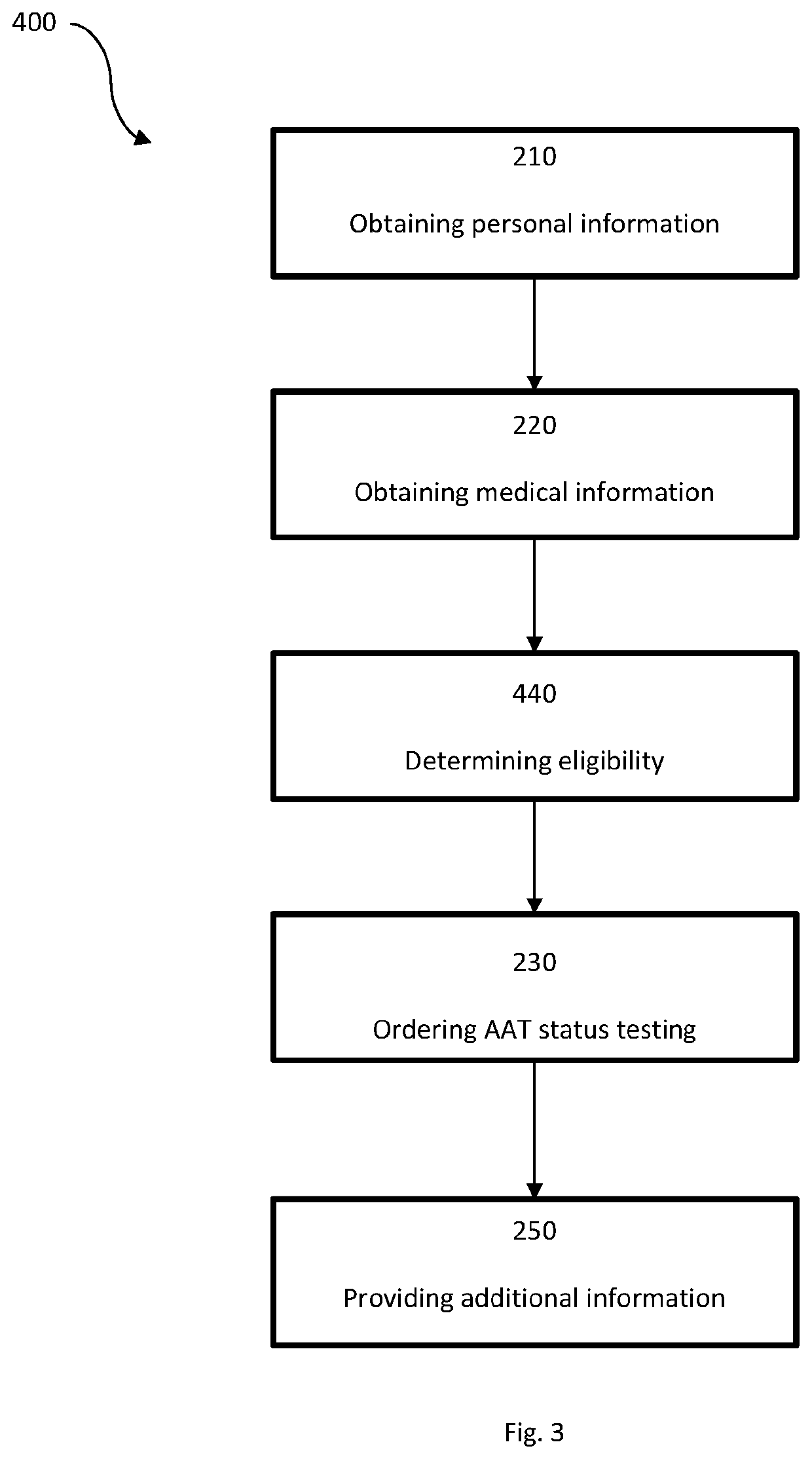
System for insurance underwriting and post policy issuance action
PendingUS20220165374A1Improve the quality of lifeProlong lifeFinanceHealth-index calculationAgricultural scienceMedicine
A system for insurance underwriting and post policy issuance action comprising obtaining personal, medical, and genetic information for an applicant and determining the applicant's eligibility for an insurance policy is provided. The applicant may additionally be provided with information regarding genetic testing for alpha-1 antitrypsin deficiency or with information regarding testing of circulating AAT levels. Furthermore, alpha-1 antitrypsin testing may be ordered for the applicant as part of the insurance underwriting process or for the insured following policy acceptance or issuance.
Owner:EGLY MARK
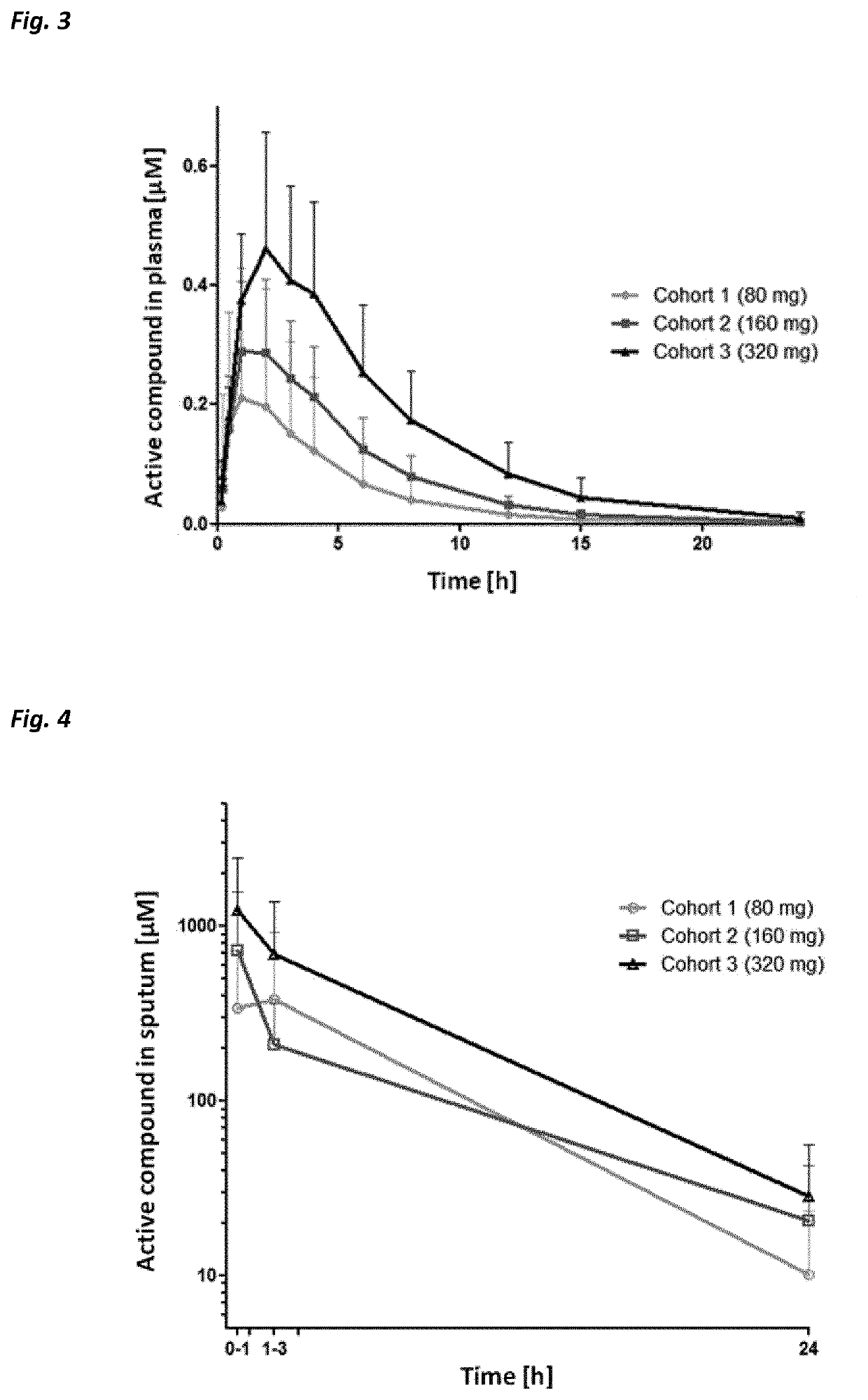
Beta-hairpin peptidomimetic with elastase inhibitory activity and aerosol dosage forms thereof
The present invention relates to pharmaceutical aerosols comprising a β-hairpin peptidomimetic of formula cyclo(-OctG-Glu-Thr-Ala-Ser-Ile-Pro-Pro-Gln-Lys-Tyr-DPro-Pro-), or a pharmaceutically acceptable salt thereof, having inhibitory activity against human neutrophil elastase. It further relates to solid or liquid pharmaceutical compositions and kits for preparing and administering such aerosols. The invention can be used for the prevention, management or treatment of pulmonary diseases, such as alpha-1 antitrypsin deficiency (AATD), cystic fibrosis (CF), non-cystic fibrosis bronchiactasis (NCFB), or chronic obstructive pulmonary disease (COPD), or infections, or diseases, or conditions of the lungs, being mediated by or resulting from human neutrophil elastase activity. Thus, the invention further relates to a pharmaceutical composition or a pharmaceutical aerosol comprising the active compound cyclo(-OctG-Glu-Thr-Ala-Ser-Ile-Pro-Pro-Gln-Lys-Tyr-DPro-Pro-), or any pharmaceutically acceptable salt thereof, for use in a method for the prevention, management or treatment of diseases or conditions of the lungs being mediated by or resulting from human neurophil elastase activity in a subject.
Owner:POLYPHOR AG
Beta-hairpin peptidomimetic with elastase inhibitory activity and aerosol dosage forms thereof
The present invention relates to pharmaceutical aerosols comprising a β-hairpin peptidomimetic of formula cyclo(-OctG-Glu-Thr-Ala-Ser-Ile-Pro-Pro-Gln-Lys-Tyr-DPro-Pro-), or a pharmaceutically acceptable salt thereof, having inhibitory activity against human neutrophil elastase. It further relates to solid or liquid pharmaceutical compositions and kits for preparing and administering such aerosols. The invention can be used for the prevention, management or treatment of pulmonary diseases, such as alpha-1 antitrypsin deficiency (AATD), cystic fibrosis (CF), non-cystic fibrosis bronchiactasis (NCFB), or chronic obstructive pulmonary disease (COPD), or infections, or diseases, or conditions of the lungs, being mediated by or resulting from human neutrophil elastase activity. Thus, the invention further relates to a pharmaceutical composition or a pharmaceutical aerosol comprising the active compound cyclo(-OctG-Glu-Thr-Ala-Ser-Ile-Pro-Pro-Gln-Lys-Tyr-DPro-Pro-), or any pharmaceutically acceptable salt thereof, for use in a method for the prevention, management or treatment of diseases or conditions of the lungs being mediated by or resulting from human neutrophil elastase activity in a subject.
Owner:POLYPHOR AG
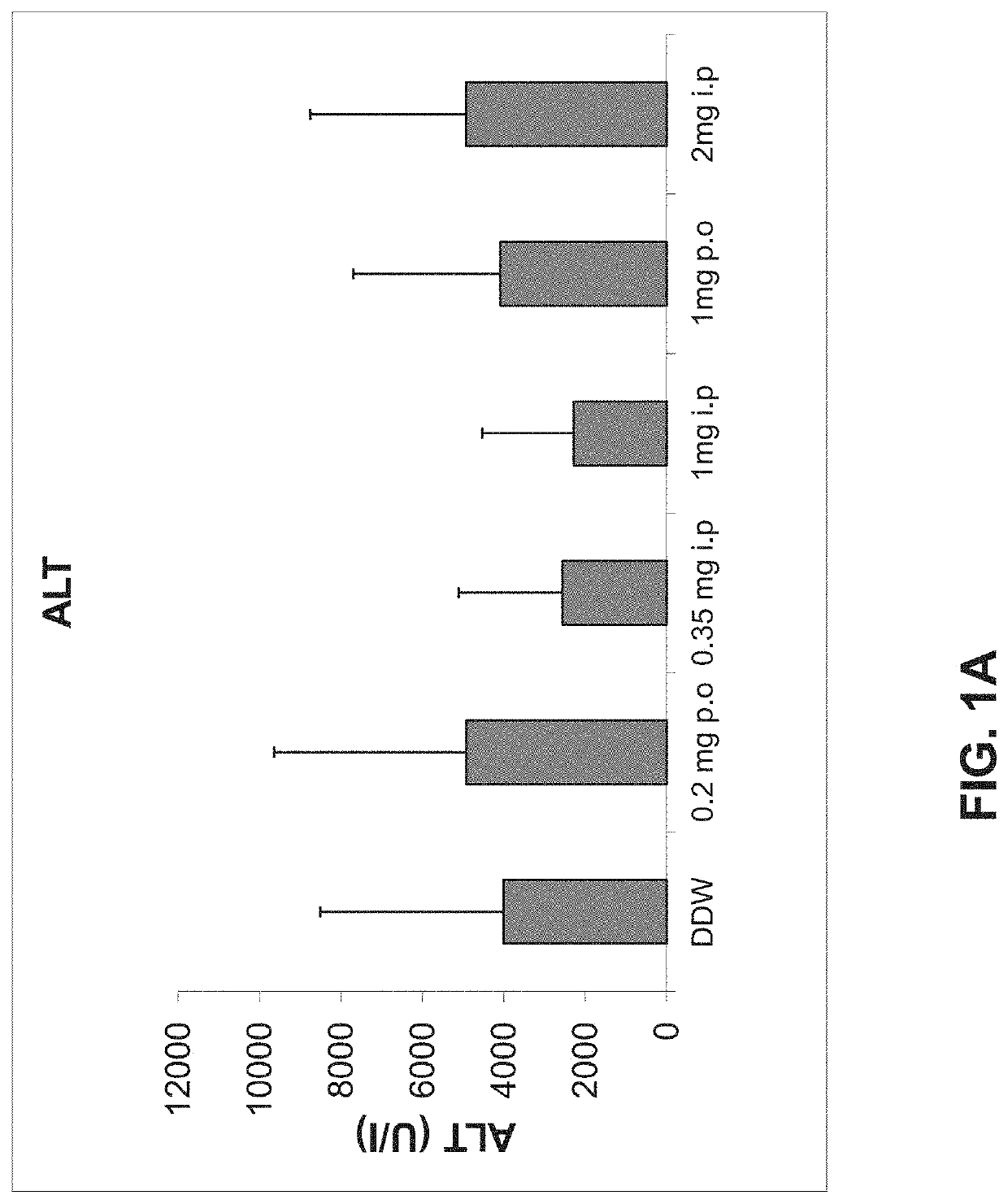
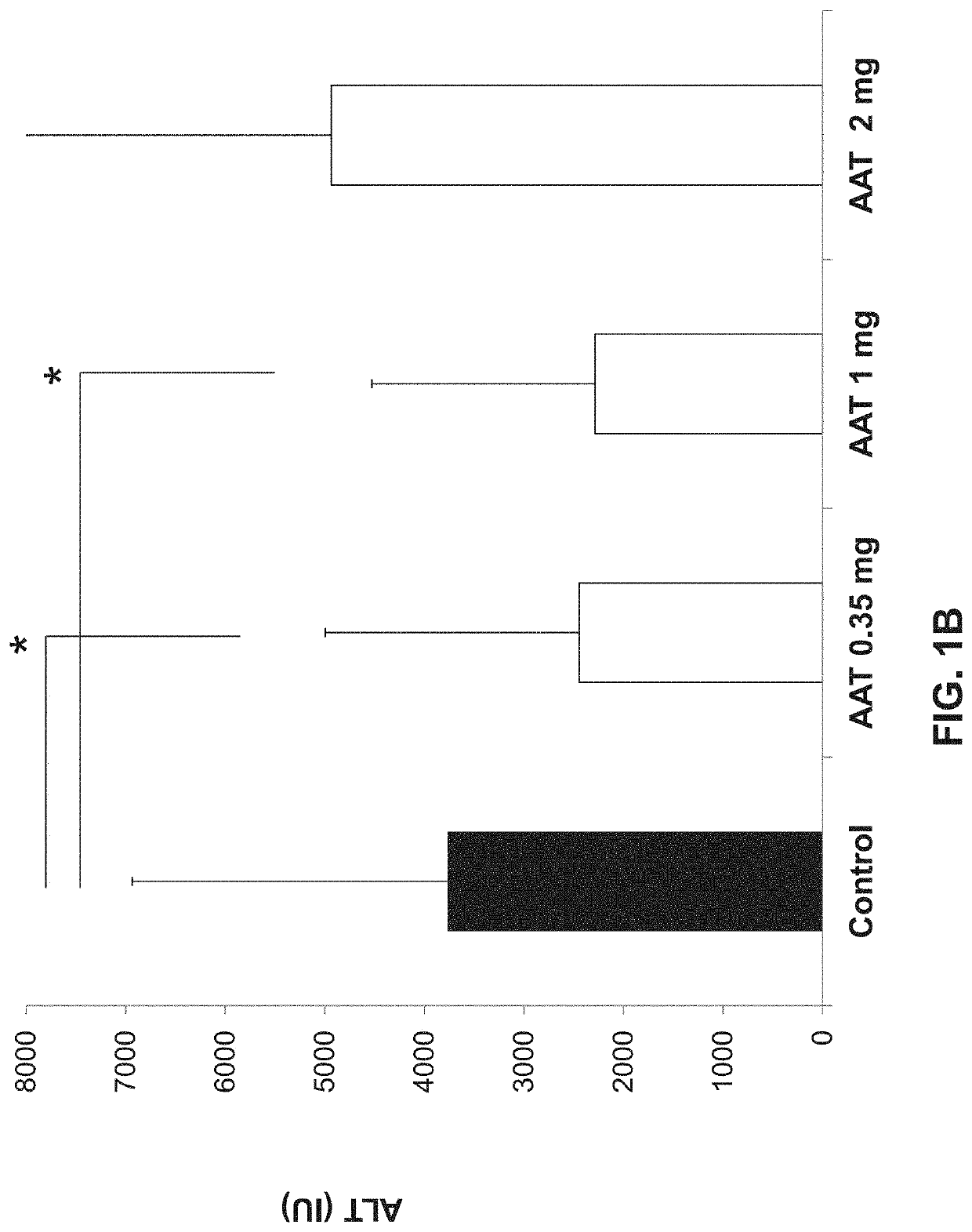
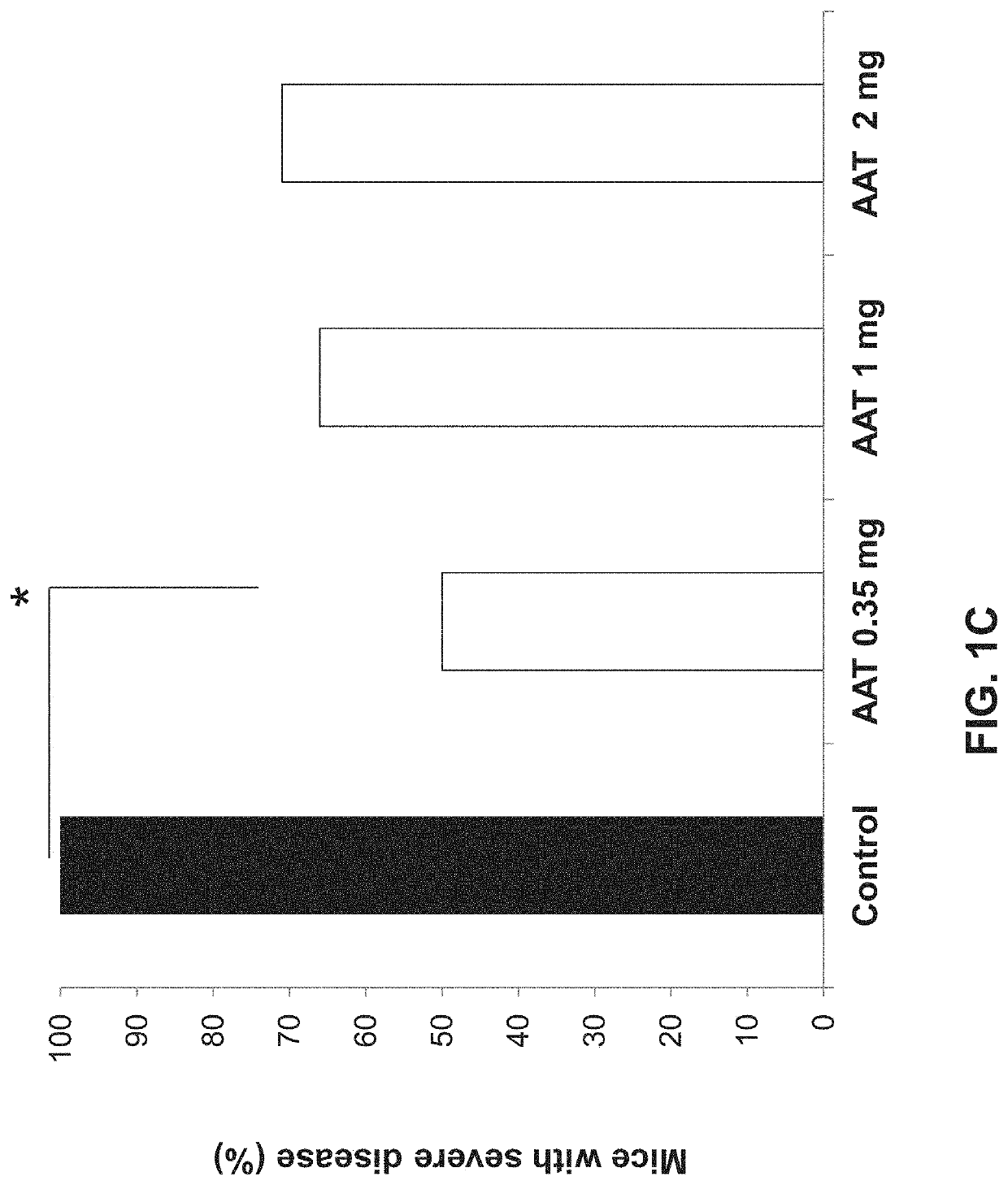
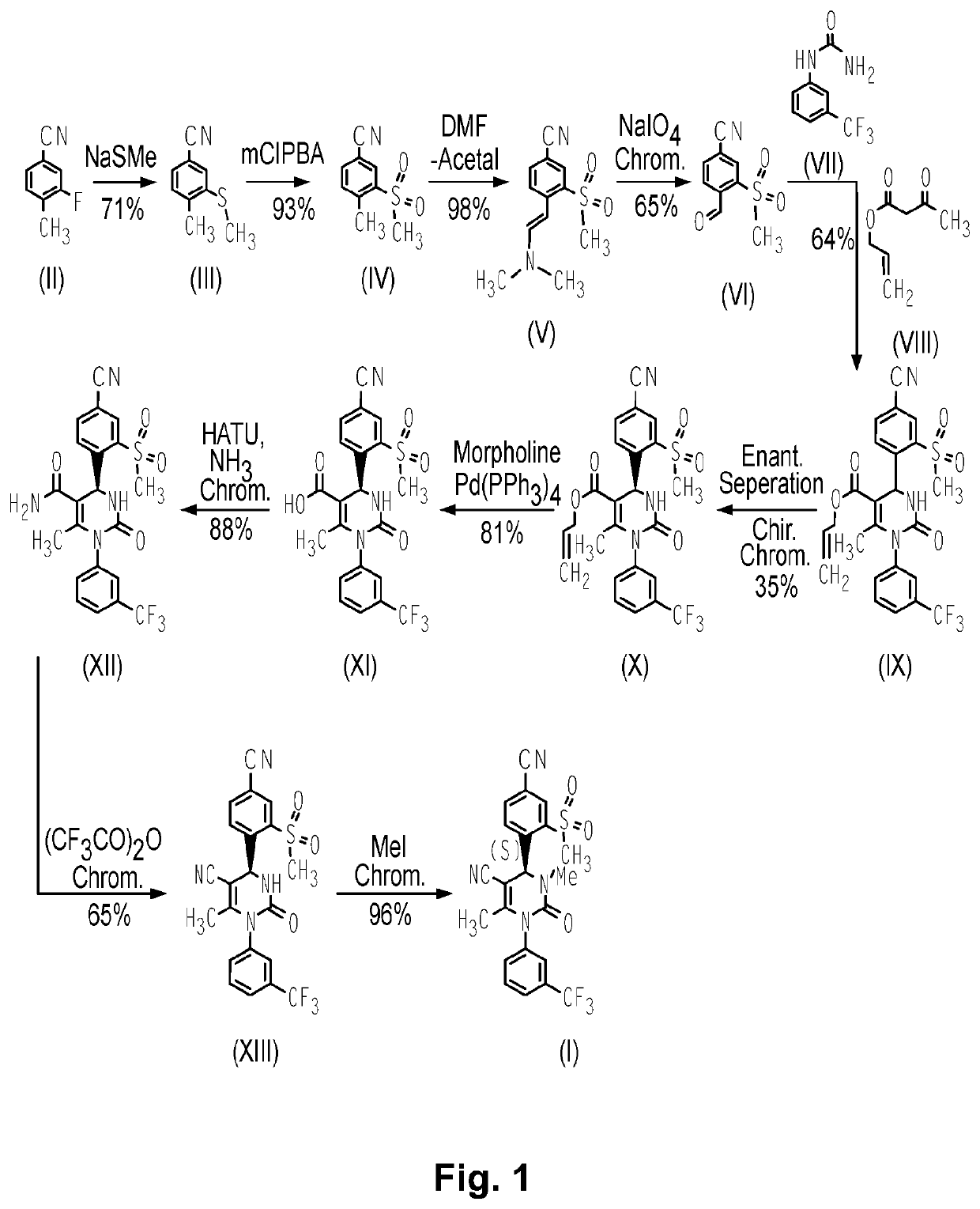
Alpha-1 anti-trypsin for treating liver diseases
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT
Use of a neutrophil elastase inhibitor in lung disease
The invention relates to methods for treating chronic lung disease, in particular, alpha-1 antitrypsin deficiency or emphysema resulting from alpha-1 antitrypsin deficiency, with a neutrophil elastase inhibitor. The invention further relates to pharmaceutical compositions comprising a neutrophil elastase inhibitor.
Owner:PH PHARMA CO LTD
Methods for treating alpha-1 antitrypsin deficiency (AATD)
PendingCN114222820ASlow or stop progressOrganic active ingredientsGenetic material ingredientsDiseaseIncreased hepatocellular carcinoma risk
Methods of treating alpha-1 antitrypsin deficiency (AATD) in a human patient in need thereof using a pharmaceutical composition comprising an AAT RNAi agent are described. The pharmaceutical compositions comprising AATRNAi agents disclosed herein treat liver diseases associated with AAT deficiency, such as chronic hepatitis, cirrhosis, increased risk of hepatocellular carcinoma, elevated transaminase, cholestasis, fibrosis, outbreak liver failure, and other liver-related diseases when administered to a human patient in need thereof.
Owner:ARROWHEAD RES CORP
Chimeric dystrophin-VSV-G protein to treat dystrophinopathies
InactiveUS9926352B2Polypeptide with localisation/targeting motifPeptide/protein ingredientsDiseaseMelanoma
Owner:OPAL IP LTD
Neutrophil inflammation inhibitor and uses thereof
ActiveUS20200148674A1Ameliorate mitigate prevent symptomTreatment and of subjectOrganic chemistryRespiratory disorderArthritisObstructive chronic bronchitis
Disclosed herein are compounds of formula (I), and pharmaceutical compositions comprising the same. The compounds of formula (I) are neutrophilic inflammation inhibitors, thus, they are useful for treatment and / or prophylaxis of inflammatory diseases and / or disorders associated with abnormal activation of neutrophils, such as ARDS, ALI, COPD, lung fibrosis, chronic bronchitis, pulmonary emphysema, α-1 anti-trypsin deficiency, cystic fibrosis, idiopathic pulmonary fibrosis, liver injury, steatohepatitis, liver fibrosis, damages caused by ischemia and reperfusion, myocardial infarction, shock, stroke, and organ transplantation, ulcerative cholitis, vasculitis, SLE, sepsis, SIRS, arthritis, psoriasis, atopic dermatitis, and inflammatory skin diseases.
Owner:CHANG GUNG UNIVERSITY OF SCIENCE AND TECHNOLOGY +2
A kit for screening genetic liver diseases
ActiveCN108913761BInterpretation is clear and objectiveHigh detection throughputMicrobiological testing/measurementNPC1Bile acid synthesis
A kit for screening genetic liver diseases with high detection throughput, high sensitivity and strong specificity, the genetic liver diseases include progressive familial intrahepatic cholestasis, benign recurrent intrahepatic cholestasis, Congenital defects in bile acid synthesis, primary bile acid malabsorption, Dubin‑Johnson syndrome, hereditary hemochromatosis, alpha 1 antitrypsin deficiency, glycogen storage disease, autosomal recessive polycystic kidney disease, Budd Chiari syndrome , glycogen accumulation disease, etc. The genetic liver disease screening detects a total of 39 genes, namely ATP8B1, ABCB11, ABCB4, TJP2, NR1H4, HSD3B7, AKR1D1, CYP7B1, AMACR, ABCD3, SLC10A2, ABCC2, HFE, TFR2, SERPINA1, G6PC, SLC37A4, AGL, PYGL, SLC40A1, PKHD1, F5, GYS2, VIPAS39, SLC25A13, JAG1, NOTCH2, PHKA2, PHKB, PHKG2, PHKA1, ALAD, HAMP, HFE2, SMPD1, ATP7B, ABCA1, NPC2, NPC1; the kit includes targeted capture probes SEQ NO: 1 to SEQ NO: 722 for all exons of 39 genes. The invention is used for gene detection.
Owner:施军平 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com