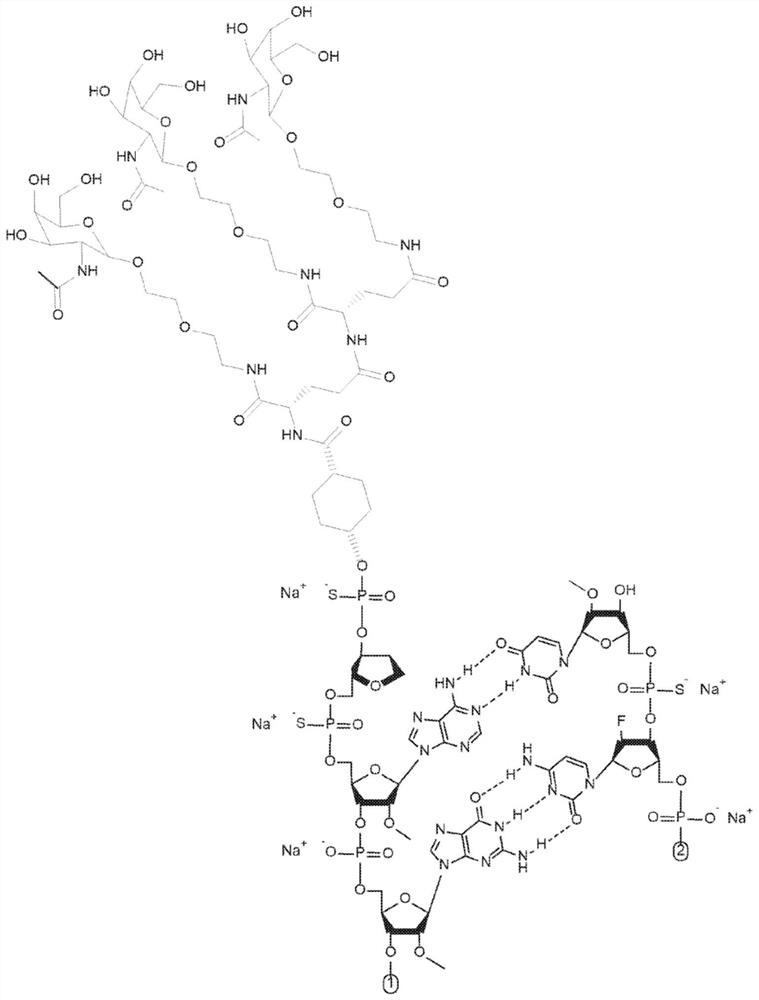
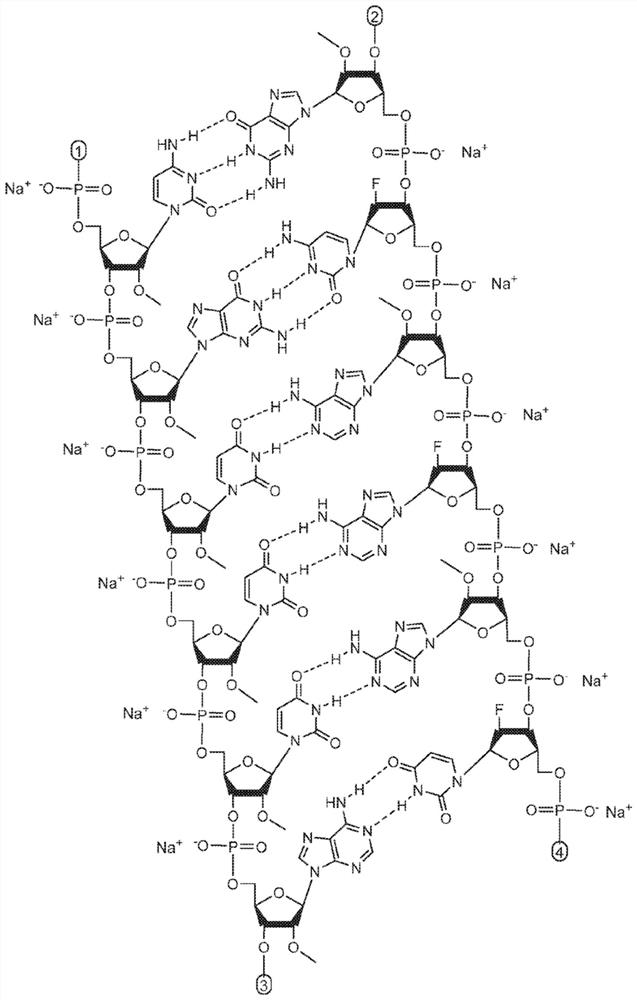
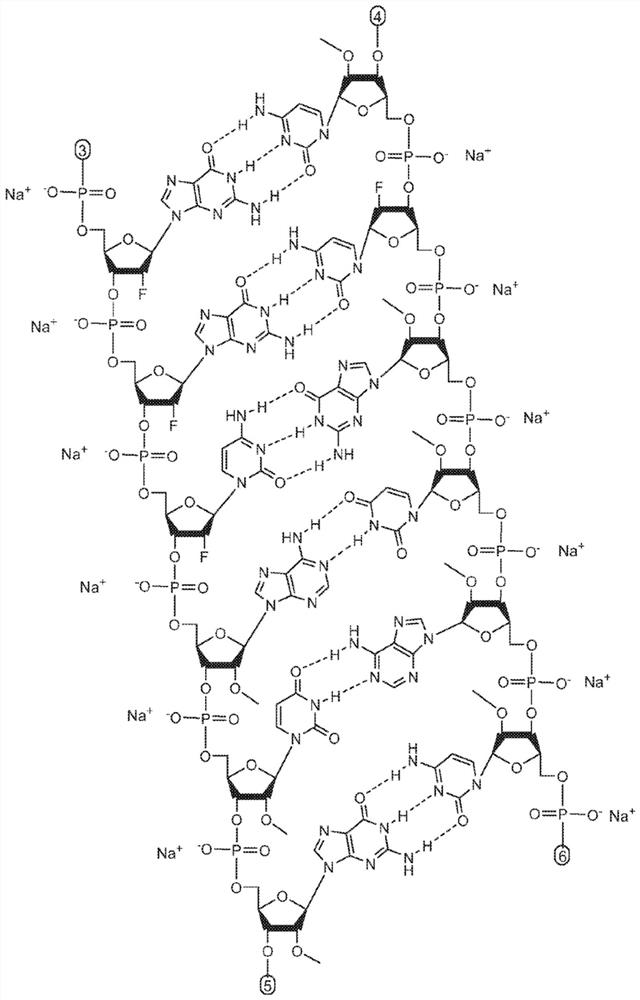
Methods for treating alpha-1 antitrypsin deficiency (AATD)
A technology for antitrypsin and deficiency, applied in gene therapy, biochemical equipment and methods, DNA/RNA fragments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Example 1. Synthesis and formulation of AAT RNAi drug substance (ADS-001)
[0137] AAT RNAi drug substances suitable for use in the methods disclosed herein can be synthesized as known in the art using standard phosphoramidite techniques based on solid phase oligonucleotide synthesis. A commercially available oligonucleotide synthesizer (e.g., (Bioautomation) or (Bioautomation)). Available in controlled pore glass (CPG, or Synthesis was performed on solid supports made from Prime Synthesis, Aston, PA, USA). The monomer at the 3' end of the corresponding chain can be attached to a solid support as the starting point for the synthesis. All RNAs, 2'-modified RNA phosphoramidites and inverted abasic phosphoramidites are commercially available. Targeting group-containing phosphoramidites suitable for addition to the 5' end of the sense strand can be synthesized. Standard cleavage, deprotection, purification and annealing steps may be utilized as known in the art. ...
Embodiment 2
[0138] Example 2. Phase I clinical trial of AAT RNAi drug substance (ADS-001) in normal healthy human volunteers (NHV).
[0139] A phase 1 single and multiple dose escalation study was conducted to evaluate the safety, tolerability, pharmacokinetics and effects of an AAT RNAi drug substance (ADS-001) on serum AAT levels in healthy volunteers (NHV). The study subject population includes BMI at 19.0kg / m 2 with 35.0kg / m 2 Healthy adult men and women between the ages of 18-52.
[0140] NHV subjects were divided into a total of seven cohorts. Cohorts 1 to 4 were randomly assigned to receive single ascending doses of 35 mg (cohort 1 ) and multiple ascending doses of 100 mg (cohort 2 ), 200 mg (cohort 3 ) and 300 mg (cohort 3 ) administered as a subcutaneous injection. 4) AAT RNAi drug substance or placebo (4 groups active: 4 groups placebo). Cohorts 1 to 4 were double-blind. Cohorts 2b, 3b and 4b were open label consisting of 4 subjects receiving single doses of 100, 200 and 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com