Pharmaceutical composition for treating and preventing respiratory tract pathogen infection and application
A pathogenic infection and respiratory technology, applied in the field of respiratory diseases, can solve problems such as the lack of vaccines on the market, achieve good application prospects, improve lung lesions, and increase survival rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] This example provides an in vitro antiviral validation experiment of rotenone.
[0052] The HRSV low-virulence strain HRSV-A-GZ08-0 and high-virulence strain HRSV-A-GZ08-18 used were referred to Zhang K, LiC, Luo Y S, et al.Establishment of a lethal aged mouse model of humanrespiratory syncytial virus infection. Antiviral Research, 2019, 161:125-133.
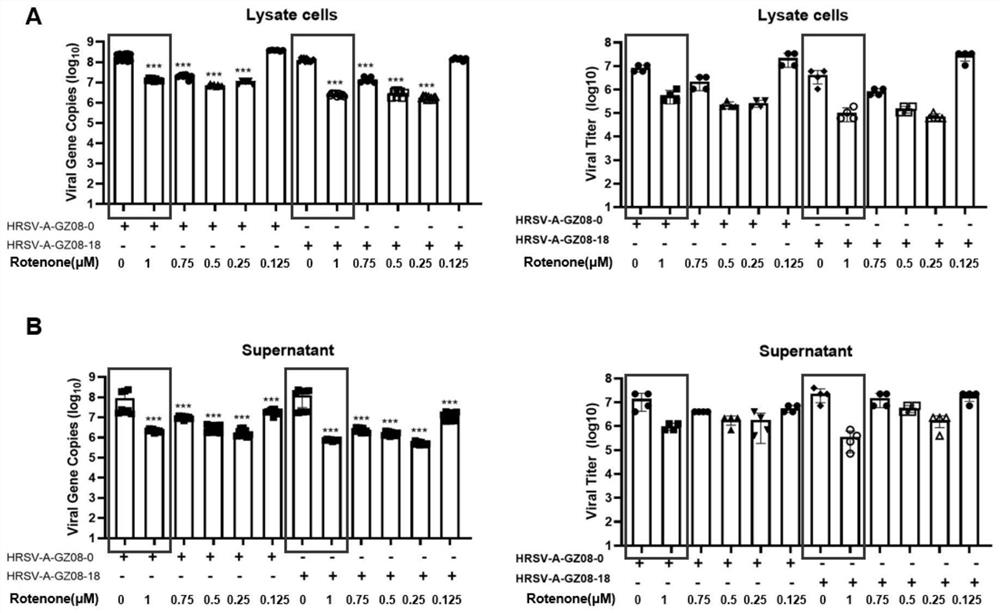
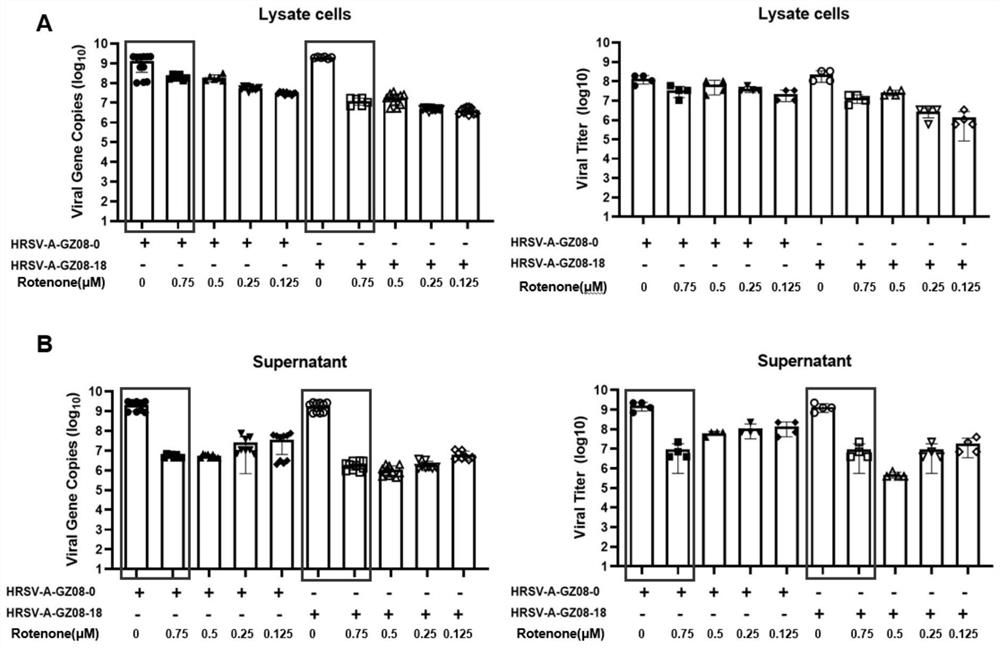
[0053] In vitro antiviral experiment: We infected HEp-2 cells with HRSV low-virulence strain HRSV-A-GZ08-0 and high-virulence strain HRSV-A-GZ08-18 for 1 hour, and then changed the medium to contain 1 μM, 0.75 μM, 0.5 μM , 0.25μM and 0.125μM concentration of rotenone in cell culture medium (DMEM-F12 / GlutaMax-I, 10565, Gibco; 2% FBS; 1% P / S) continued to culture, followed by TCID at 48 hours and 72 hours after infection, respectively 50 Virus titers in cell pellets and cell supernatants were determined and compared with real-time quantitative PCR.
[0054] result reference figure 1 and figure 2 shown, visible 48 hours...
Embodiment 2
[0060] In this example, the in vivo antiviral verification experiment of rotenone was carried out.
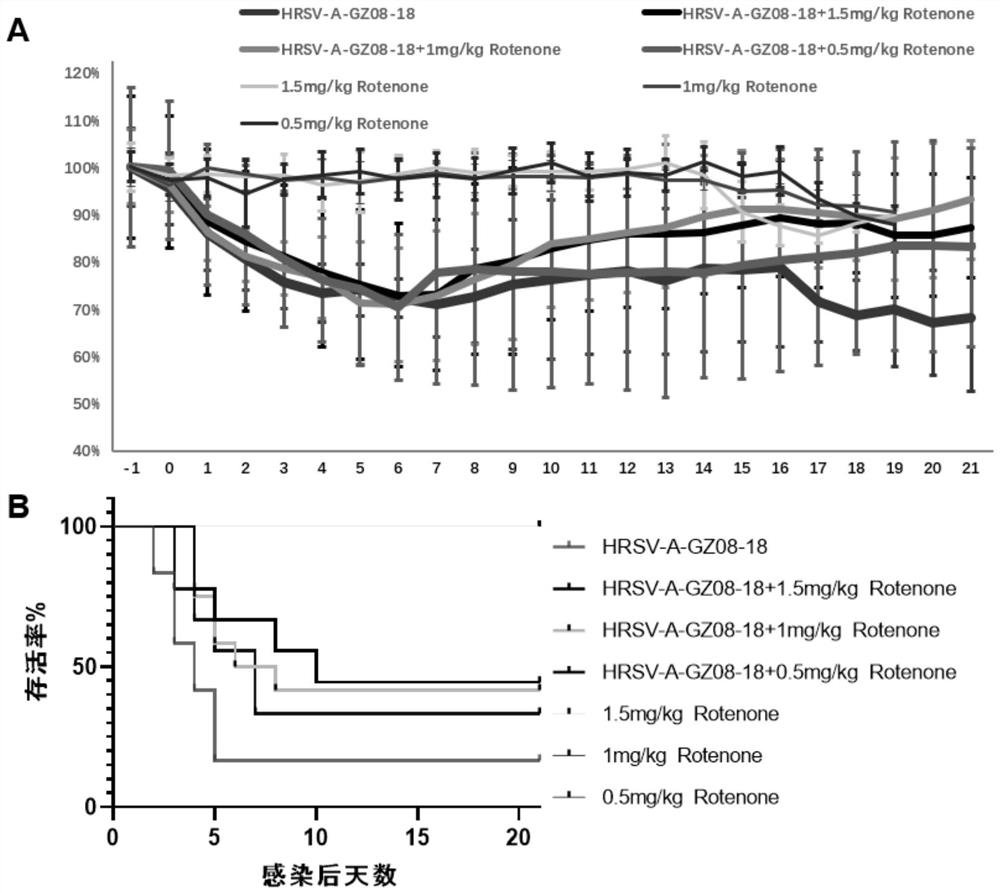
[0061] After infecting BALB / c mice with HRSV high-virulence strain HRSV-A-GZ08-18 (the same source as in Example 1), we dissolved rotenone in physiological saline at different concentrations, at 1.5 mg / kg, 1.0 mg / kg and 0.5 mg / kg. Mice were injected subcutaneously with mg / kg rotenone for 3 days, and HRSV-A-GZ08-18 virus control group and 1.5 mg / kg, 1.0 mg / kg and 0.5 mg / kg rotenone subcutaneous injection control groups were set at the same time.
[0062] Figure 5 4, 5, and 6 days after HRSV infection, the results of the measurement of mouse pneumovirus titers in the mice in the saline-treated group after HRSV infection and in the mice in the 1.0 mg / kg rotenone-treated group after HRSV infection, the measurement method is TCID 50Law. The results showed that the viral load in the lungs of mice in the saline-treated group on the 4th and 5th day after HRSV infection was significa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com