Method for synthesizing beta-myrcene through intramolecular decarboxylation allyl substitution reaction
A technology of allyl substitution and carboxyallyl, applied in the field of fine chemicals, can solve the problems of no commercial value, low energy consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment i
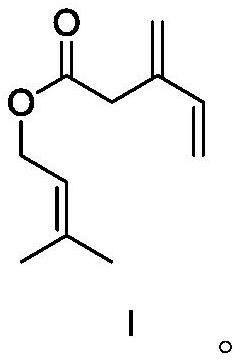
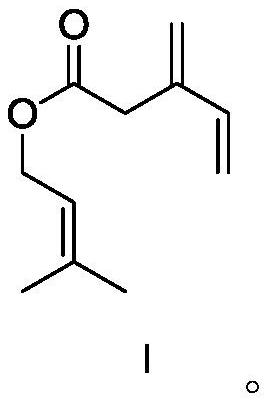
[0055] Synthesis of compound I.
[0056]
[0057] In a flask, add 3-methylene-4-enevaleric acid (1 mol), 3-methyl-2-buten-1-ol (1 mol) and 200 ml of dichloromethane, stir mechanically, and let the system cool down to After 0 °C, dicyclohexylcarbodiimide (DCC) (1.1 mol) and 4-dimethylaminopyridine (DMAP) (0.2 mol) were added to the system, then the temperature was raised to room temperature, and the reaction was continued to stir for 12 h to stop the reaction. The solid was filtered off with celite, and the filtrate was washed with 5% HCl, saturated NaHCO successively 3 It was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed in vacuo. The residue was separated by silica gel column chromatography to obtain product I (yield 94%). 1 H NMR (400MHz, C 6 D 6 ): δ1.69(s, 3H), 1.78(s, 3H), 3.58(s, 2H), 4.48(d, 2H), 4.79–5.01(m, 2H), 5.10–5.20(m, 2H), 5.35 (m, 1H), 6.24 (m, 1H).
[0058] Example ii
[0059] Synthesis of compound I....
Embodiment iv
[0065] Synthesis of compound I.
[0066] In a flask, add 3-methylene-4-enevaleric acid (1 mol), 3-methyl-2-buten-1-ol (1.2 mol) and 200 ml of dichloromethane, stir mechanically, and wait for the system to cool After reaching 0 °C, dicyclohexylcarbodiimide (DCC) (1.1 mol) and 4-dimethylaminopyridine (DMAP) (0.2 mol) were added to the system, then the temperature was raised to room temperature, and the reaction was continued for 1 h to stop the reaction. , the solid was filtered off with celite, and the filtrate was successively washed with 5% HCl, saturated NaHCO 3 It was washed with saturated brine, dried over anhydrous sodium sulfate, the solvent was removed in vacuo, and the residue was isolated by silica gel column chromatography to obtain product I (yield 83%).
Embodiment v
[0068] Synthesis of compound I.
[0069] In a flask, add 3-methylene-4-enevaleric acid (1 mol), 3-methyl-2-buten-1-ol (1.2 mol) and 200 ml of dichloromethane, stir mechanically, and wait for the system to cool After reaching -10°C, add dicyclohexylcarbodiimide (DCC) (1.1mol) and 4-dimethylaminopyridine (DMAP) (0.2mol) to the system, then warm to room temperature, continue to stir the reaction for 12h, stop After the reaction, the solid was filtered off with celite, and the filtrate was successively washed with 5% HCl, saturated NaHCO 3 It was washed with saturated brine, dried over anhydrous sodium sulfate, the solvent was removed in vacuo, and the residue was isolated by silica gel column chromatography to obtain product I (yield 99%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com