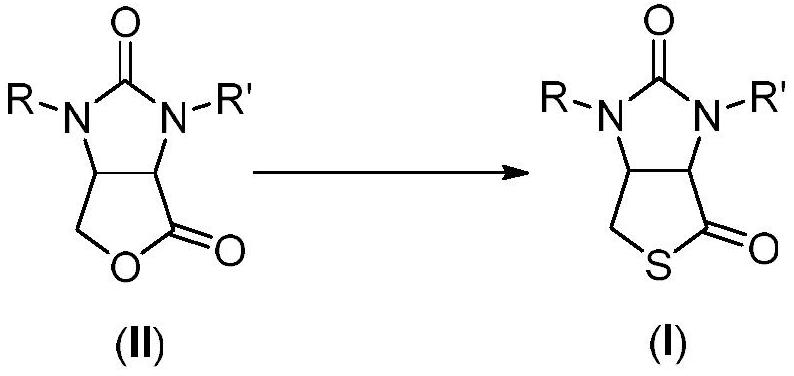
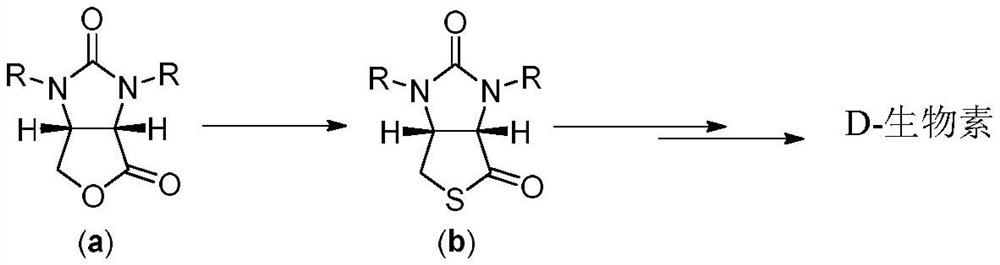
Method for preparing D-biotin intermediate
A compound and mixture technology, applied in the field of preparation of thiolactone compounds, can solve problems such as trouble, affecting the yield and purity of thiolactone compounds, instability of potassium thioacetate, etc., and achieve high-purity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] In a 250 mL four-necked flask equipped with a thermometer, stirrer, condenser and nitrogen vent and supported in a thermo-statically controlled oil bath, charged with 12.50 g of (+)-lactone 2 and 7 mL of DMF, and heated to an internal temperature of 145°C.
[0040] Under nitrogen atmosphere, 7.51 g of potassium thiobenzoate was dissolved in 17 mL of DMF. While maintaining an internal temperature of 145°C, the potassium thiobenzoate solution was quickly transferred to the flask. An additional 2 mL of DMF was added to ensure complete transfer.
[0041] After stirring at 145°C for 60 minutes, the obtained mixture was cooled to about 50°C and then 56 mL of toluene was added. After stirring for an additional 5 minutes, the mixture was transferred to a separatory funnel containing 108 mL of water. An additional 67 mL of toluene was added to complete the transfer.
[0042]The funnel containing the two layers was shaken, then the lower aqueous phase was separate...
Embodiment 2
[0044]
[0045] In a 250 mL four-necked flask equipped with a thermometer, stirrer, condenser and nitrogen vent and supported in a thermostatically controlled oil bath, 12.50 g of (+)-lactone 2 and 7 mL of NMP were charged and heated to 145°C internal temperature.
[0046] Under nitrogen atmosphere, 7.51 g of potassium thiobenzoate was dissolved in 17 mL of NMP. While maintaining an internal temperature of 145°C, the potassium thiobenzoate solution was quickly transferred to the flask. An additional 2 mL of NMP was added to ensure complete transfer.
[0047] After stirring at 145°C for 60 minutes, the obtained mixture was cooled to about 50°C and then 56 mL of toluene was added. After stirring for an additional 5 minutes, the mixture was transferred to a separatory funnel containing 108 mL of water. An additional 67 mL of toluene was added to complete the transfer.
[0048] The funnel containing the two layers was shaken, then the lower aqueous phase was separated into ...
Embodiment 3
[0050]
[0051] In a 250 mL four-necked flask equipped with a thermometer, stirrer, condenser and nitrogen vent and supported in a thermostatically controlled oil bath, charged with 12.50 g of (+)-lactone 2 and 7 mL of DEF and heated to 145°C internal temperature.
[0052] Under nitrogen atmosphere, 7.51 g of potassium thiobenzoate was dissolved in 17 mL of DEF. While maintaining an internal temperature of 145°C, the potassium thiobenzoate solution was quickly transferred to the flask. An additional 2 mL of DEF was added to ensure complete transfer.
[0053] After stirring at 145°C for 60 minutes, the obtained mixture was cooled to about 50°C and then 56 mL of toluene was added. After stirring for an additional 5 minutes, the mixture was transferred to a separatory funnel containing 108 mL of water. An additional 67 mL of toluene was added to complete the transfer.
[0054] The funnel containing the two layers was shaken, then the lower aqueous phase was separated into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com