Cobalt niobium oxygen cluster and preparation method and application thereof
An oxygen cluster and crystalline technology, applied in the field of synthesis of polyniobate, can solve the problem of easy formation of precipitation, and achieve the effects of cheap materials, simple operation and few synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
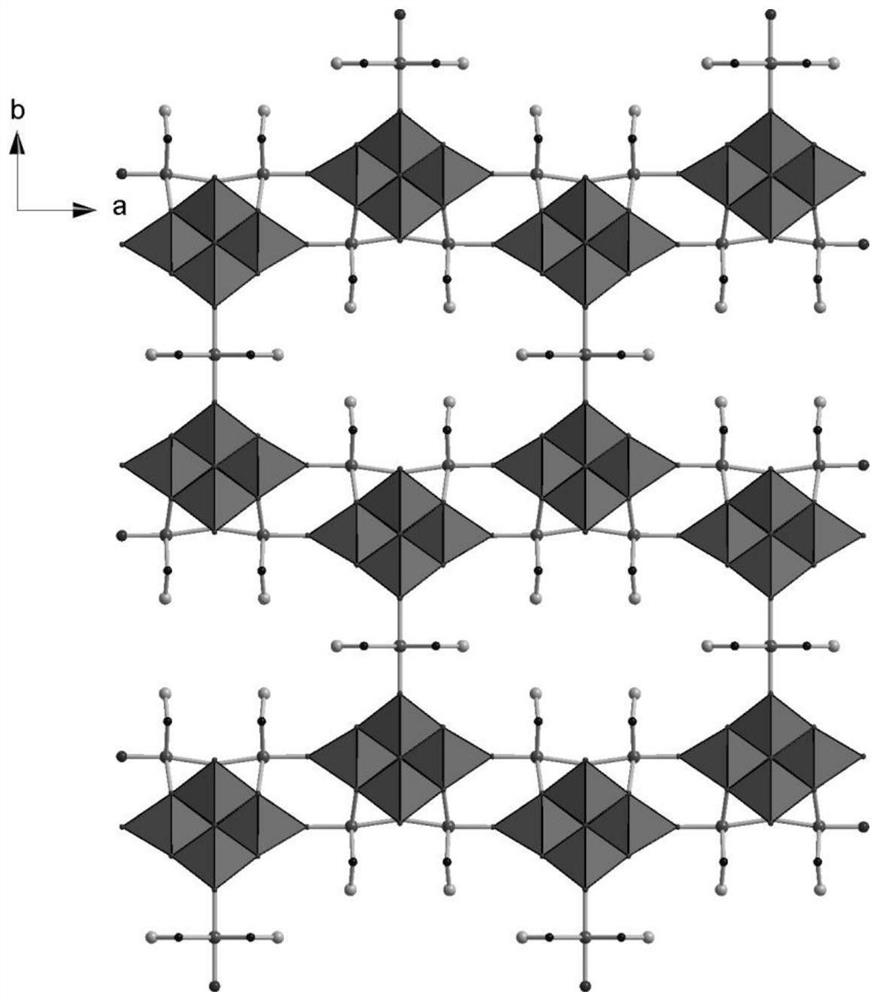
[0026] A cobalt-oxygen cluster, the cobalt-oxygen cluster is organic-inorganic hybrid cobalt-cobalt oxygen cluster crystal material. 3 [NB 6 O 19 {Co (EN)} 2 CO 0.5 (EN)] · 14H 2 O.
[0027] A method of preparing a cobalt oxygen cluster, the steps are:
[0028] Step 1: Take 0.458g K 7 HNB 6 O 19 · 13H 2 O (0.33mmol) dissolved in 8ml of water;
[0029] Step 2: Take another 0.848g sodium arsenic na 3 ASO 4 , 0.352gnaCl, 0.246G Six -hydrophilic cobalt chloride COCL 3 · 6H 2 O and 1.4ml ethyleine to add K to K 7 HNB 6 O 19 · 13H 2 O in the water solution;
[0030] Step 3: Stir for 2.5 hours, transfer to polytetrafluoroethylene reactor, put on the shell;
[0031] Step 4: 4 days at 150 ° C, then cooled to room temperature to get light red color block crystals, the crystal is NA 3 [NB 6 O 19 {Co (EN)} 2 CO 0.5 (EN)] · 14H 2 O.
Embodiment 2
[0033] A cobalt-oxygen cluster in this embodiment, the cobalt-cobalt oxygen cluster is organic-inorganic hybrid cobalt-cobalt-oxygen cluster crystal material, which is chemical for cobalt-cobalt oxygen cluster crystal materials 3 [NB 6 O 19 {Co (EN)} 2 CO 0.5 (EN)] · 14H 2 O.
[0034] A method of preparing a cobalt oxygen cluster, the steps are:
[0035] Step 1: Take 0.458g K 7 HNB 6 O 19 · 13H 2 O (0.33mmol) dissolved in 8ml of water;
[0036] Step 2: Take another 0.352G NaCl, 0.246G Six water -mortar cobalt chloride COCL 3 · 6H 2 O and 1.4ml ethyleine to add K to K 7 HNB 6 O 19 · 13H 2 O in the water solution;
[0037] Step 3: Stir for 2.5 hours, transfer to polytetrafluoroethylene reactor, put on the shell;
[0038] Step 4: 4 days at 150 ° C, then cooled to room temperature to get light red color block crystals, the crystal is NA 3 [NB 6 O 19 {Co (EN)} 2 CO 0.5 (EN)] · 14H 2 O.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com