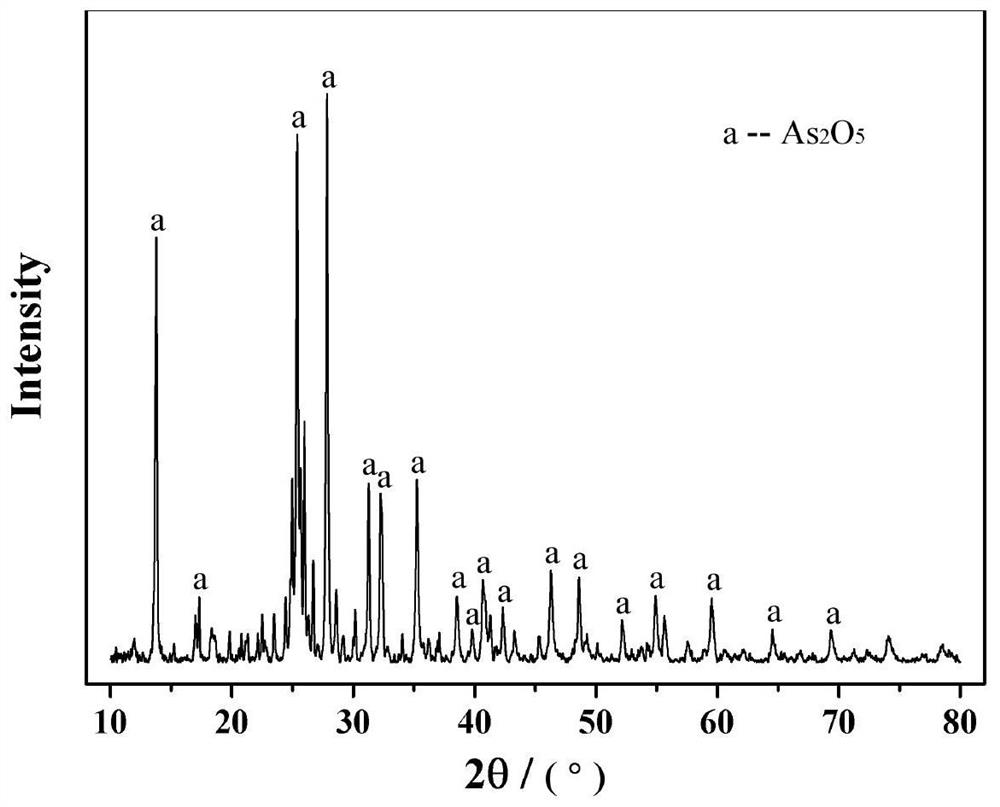
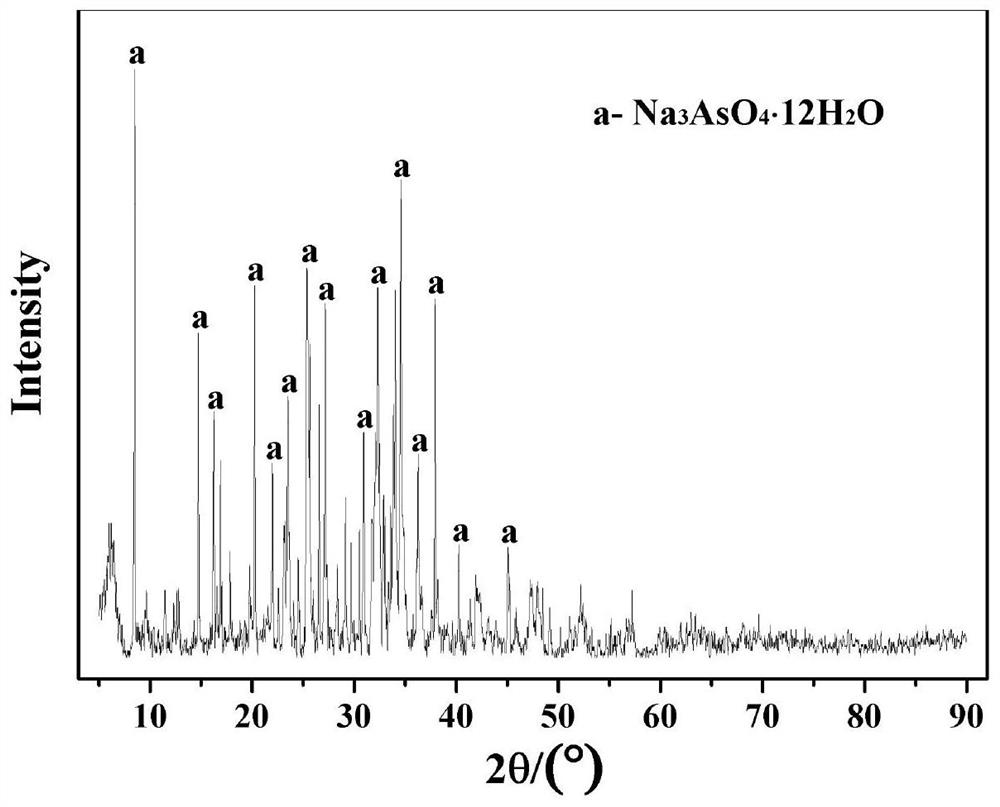
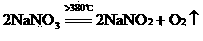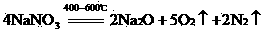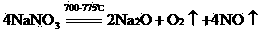Patents
Literature
43 results about "Disodium arsenate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium arsenate is the inorganic compound with the formula Na3AsO4. Related salts are also called sodium arsenate, including Na2HAsO4 (disodium hydrogen arsenate) and NaH2AsO4 (sodium dihydrogen arsenate). The trisodium salt is a white or colourless solid that is highly toxic.
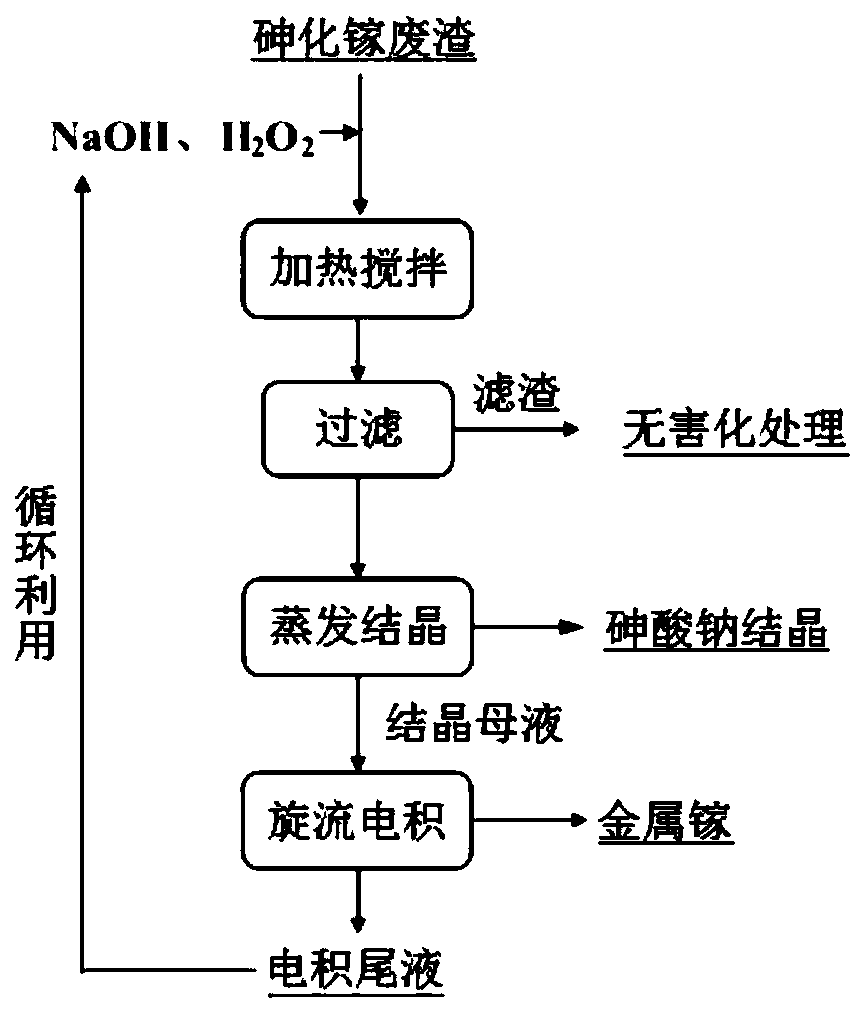
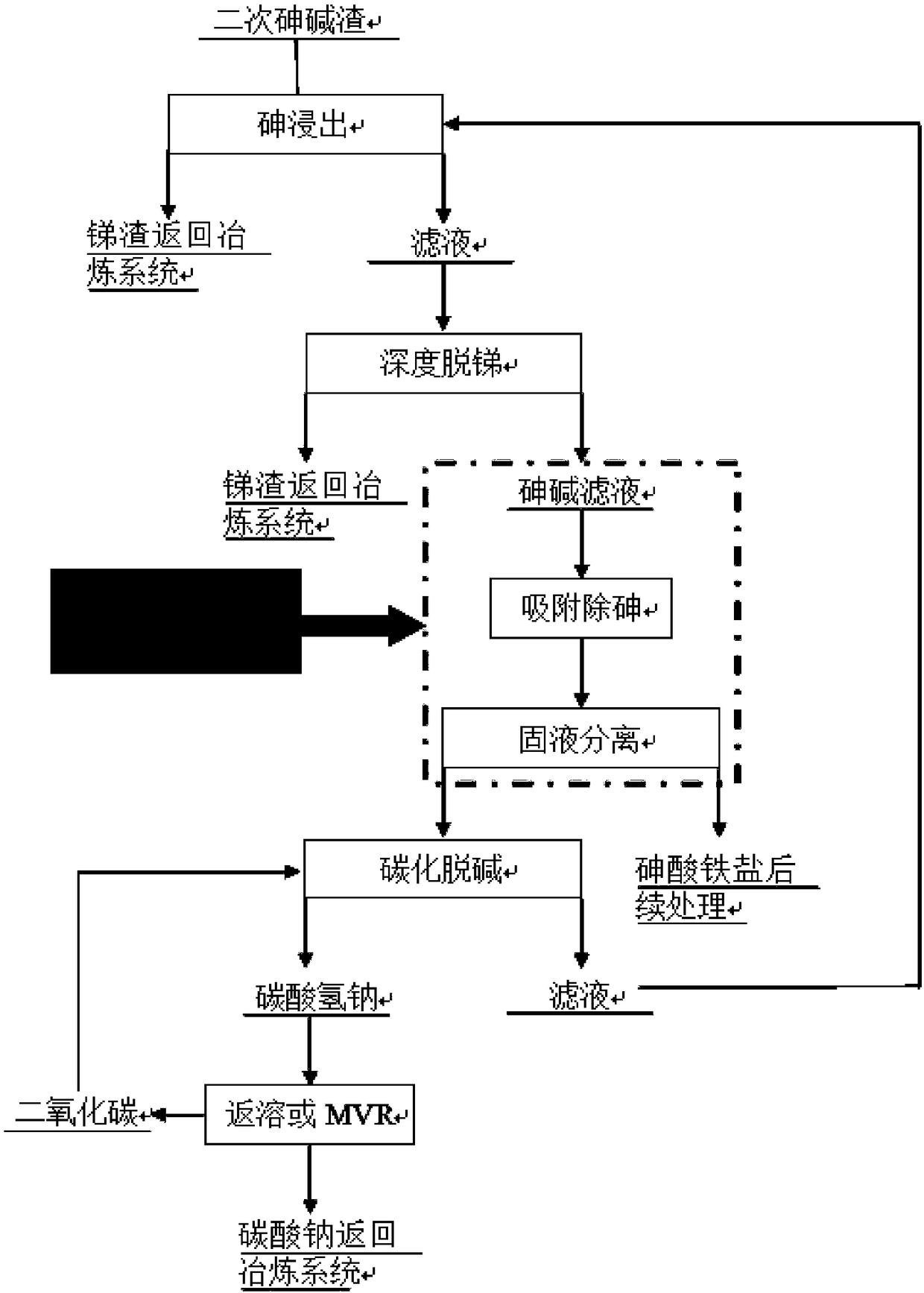
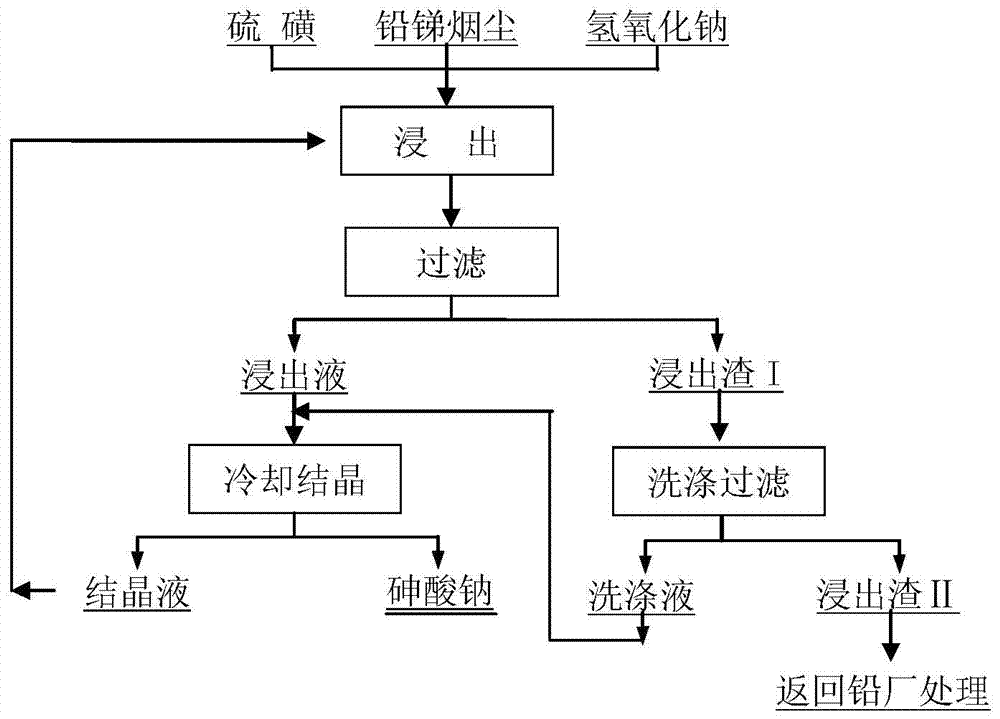
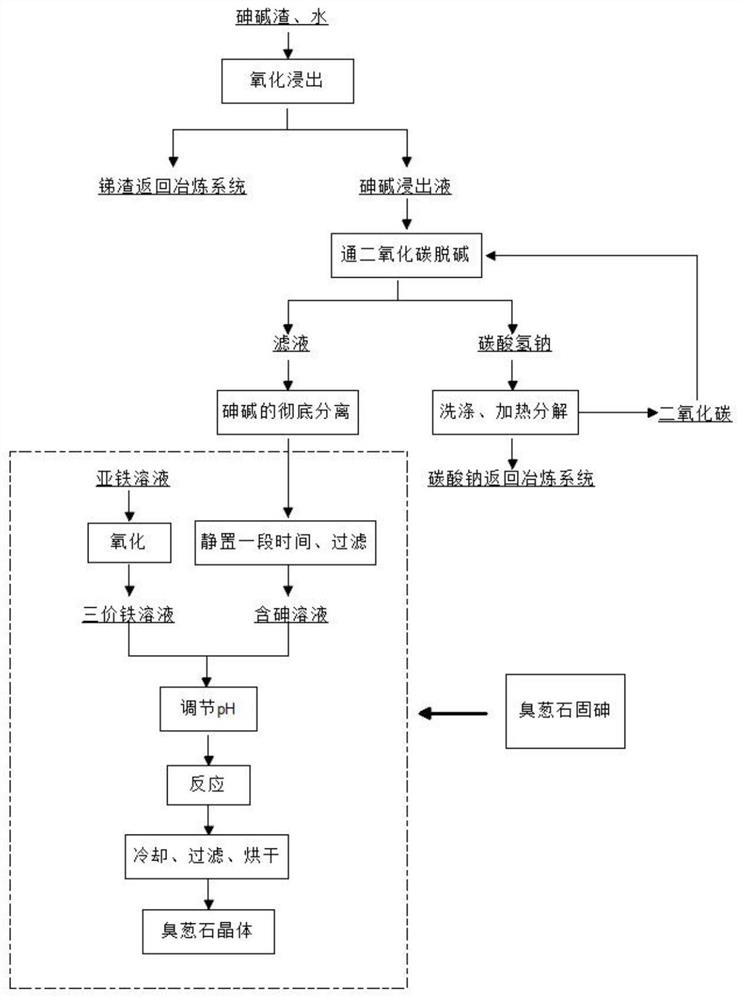
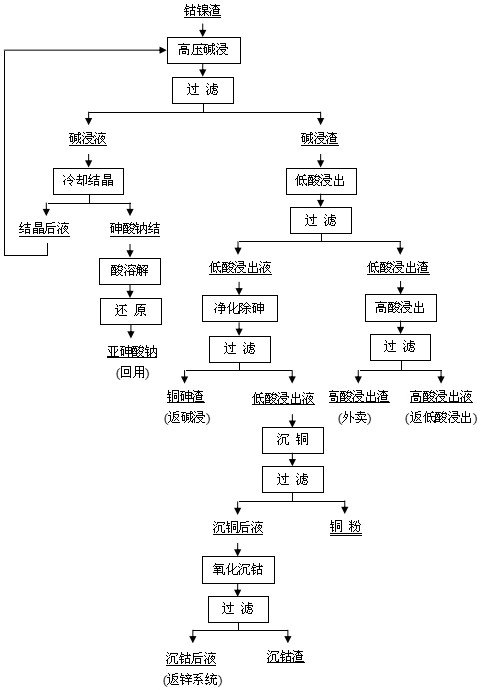
Treatment method of arsenic-containing waste
ActiveCN106180138AReduce leaching toxicityConvenient and harmless disposalSolid waste disposalWater contaminantsHigh concentrationSodium arsenite
The invention discloses a recycling treatment method of hazardous arsenic-containing waste. The recycling treatment method comprises the steps of carrying out alkaline leaching on a high-arsenic material to obtain a high-concentration sodium arsenite solution or a high-concentration sodium arsenate solution, and completely converting the high-concentration sodium arsenite solution or the high-concentration sodium arsenate solution into sodium arsenate through oxidation; adding a small amount of seed crystal-lepidocrocite (gamma-FeOOH) slurry into a reaction kettle; slowly adding an arsenic-containing solution; adding a slightly excessive high-concentration malysite solution; maintaining the reaction pH to be 3.5 to 5.5 through adding a neutralizer; and controlling the temperature in the reaction process to enable arsenic in the solution to completely react and totally deposit, wherein an obtained deposit is easy to filter, harmless and stable treatment of the arsenic-containing solution can be achieved, when the method is utilized to deposit arsenic, the concentration of arsenic in filtrate after reaction can directly reach the standard, and secondary arsenic deposition is avoided. The treatment method is simple in technological process, low in cost and applicable to industrial application.
Owner:SHENZHEN SHENTOU ENVIRONMENT TECH CO LTD
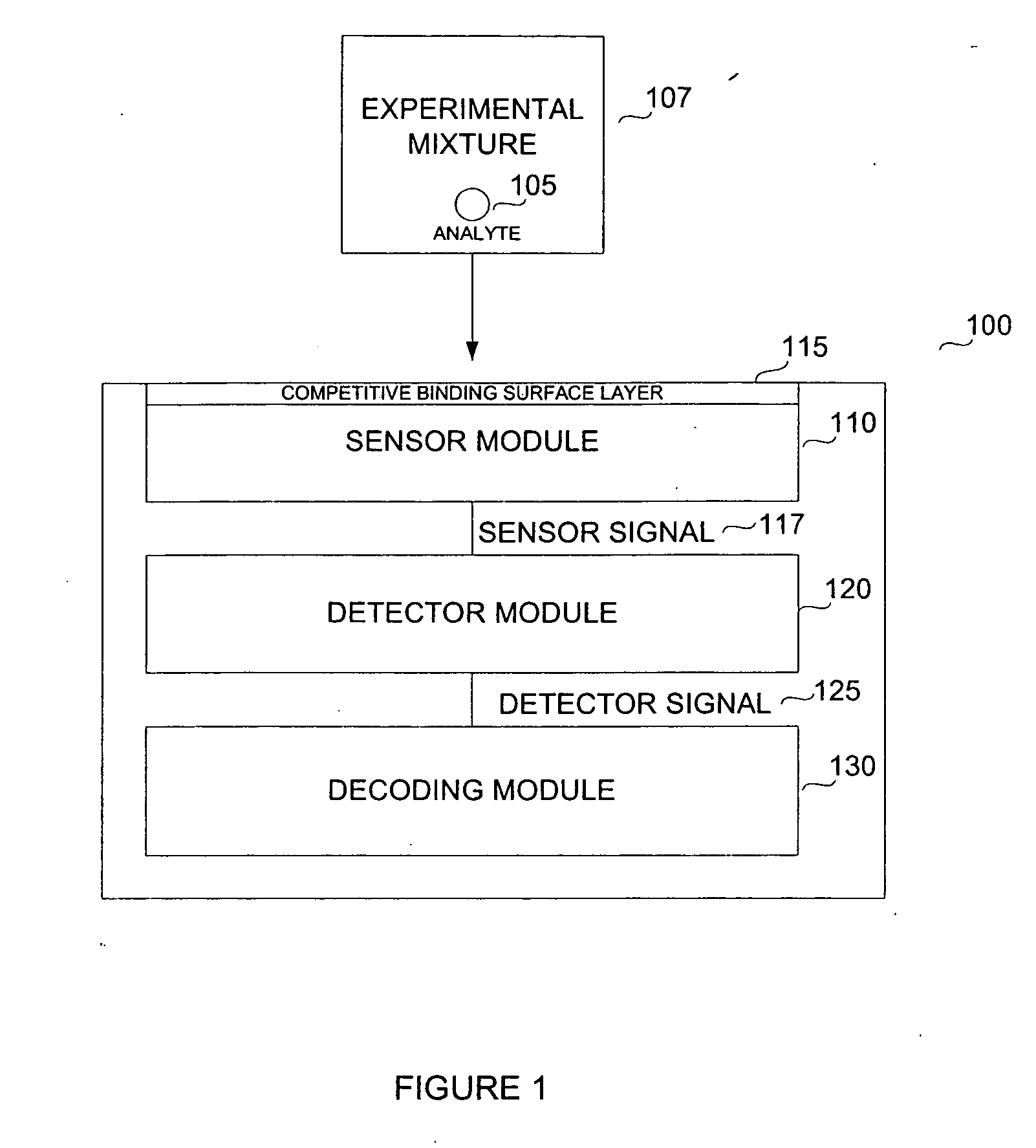
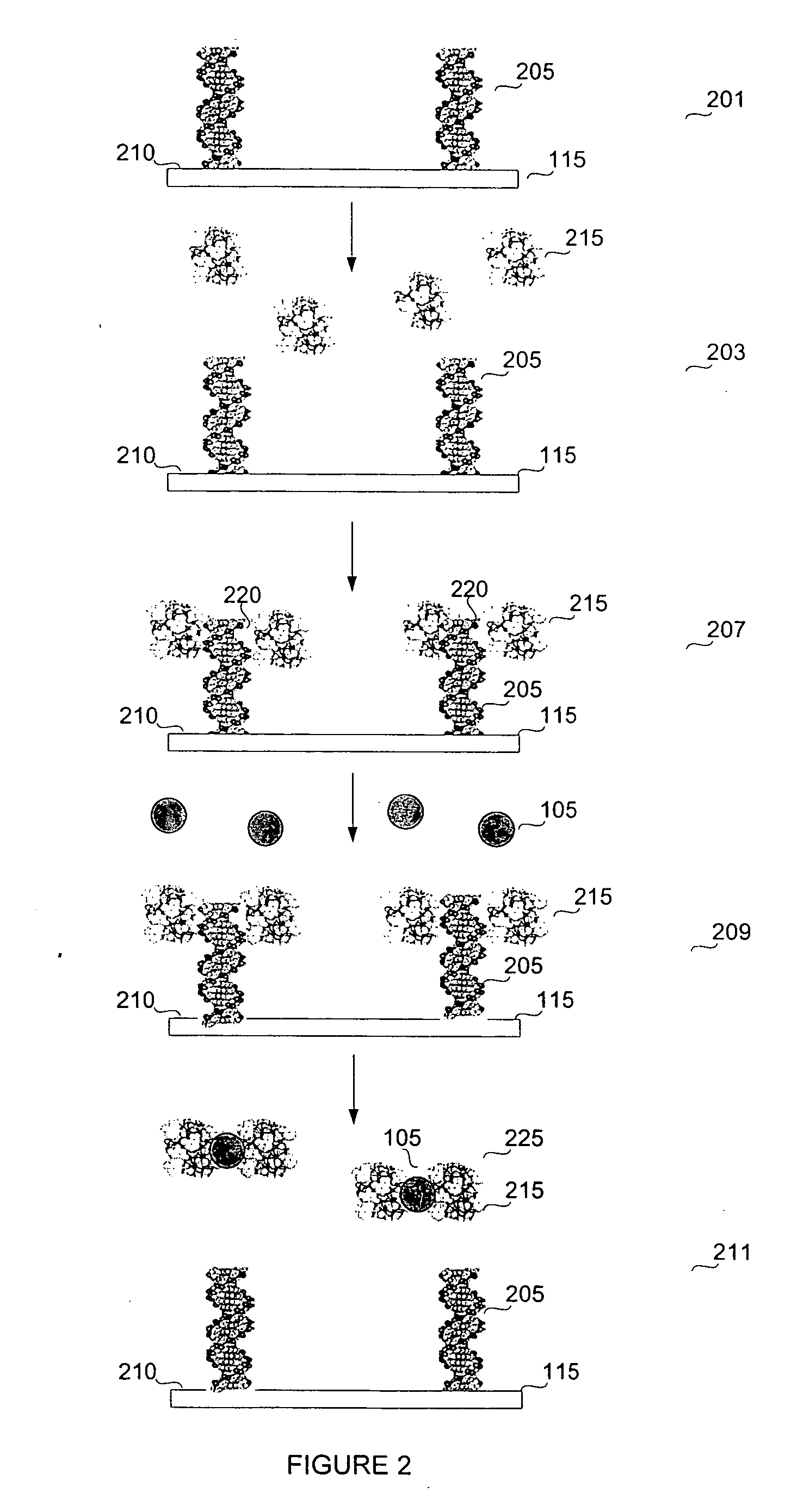
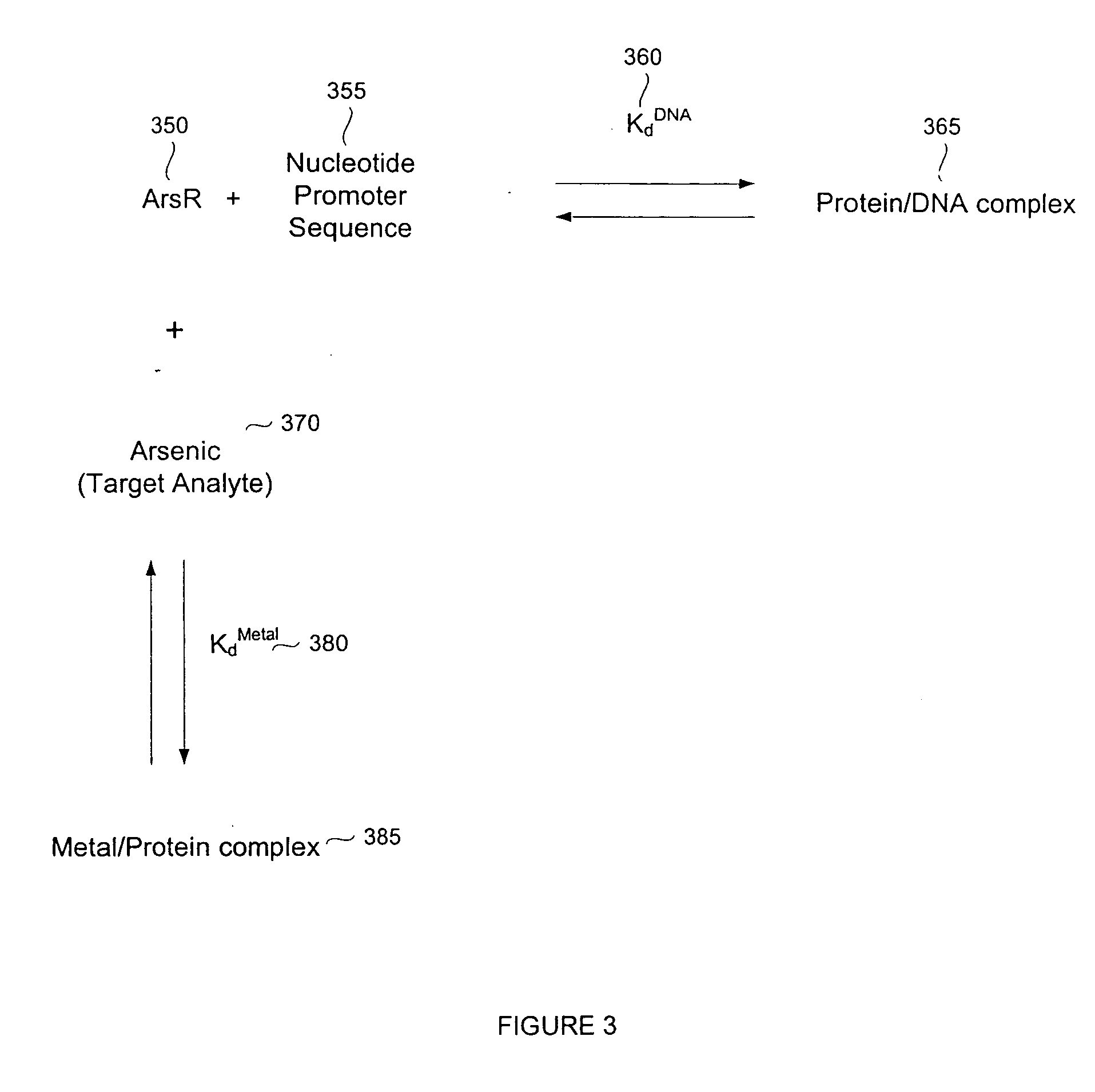
Biosensor for small molecule analytes
InactiveUS20060292581A1High sensitivityStrong specificityBioreactor/fermenter combinationsBiological substance pretreatmentsEscherichia coliPhenylarsine oxide
A biosensor device for detecting small molecules analytes is provided. The device employs a first class of molecules, e.g., protein that binds to both the analyte and a second class of molecules, e.g., nucleic acid. The binding of the protein to the analyte and nucleic acid can be mutually exclusive, and the presence of analyte in a sample results in a detectable displacement of protein from nucleic acid. Alternatively, binding of the protein to the nucleic acid can depend on the presence of analyte in the sample. In a specific embodiment, either the protein or nucleic acid is immobilized on a solid phase support. An arsenic detection system is exemplified. An ArsR binding sequence from the E. coli ars operon is immobilized on a gold-plated surface. ArsR protein binds to the DNA in the absence of arsenic, and is released in the presence of sodium arsenate or phenylarsine oxide. Protein release results in a change in surface plasmon resonance, and the magnitude or kinetics of the change indicate the concentration of arsenic.
Owner:LAING LANCE
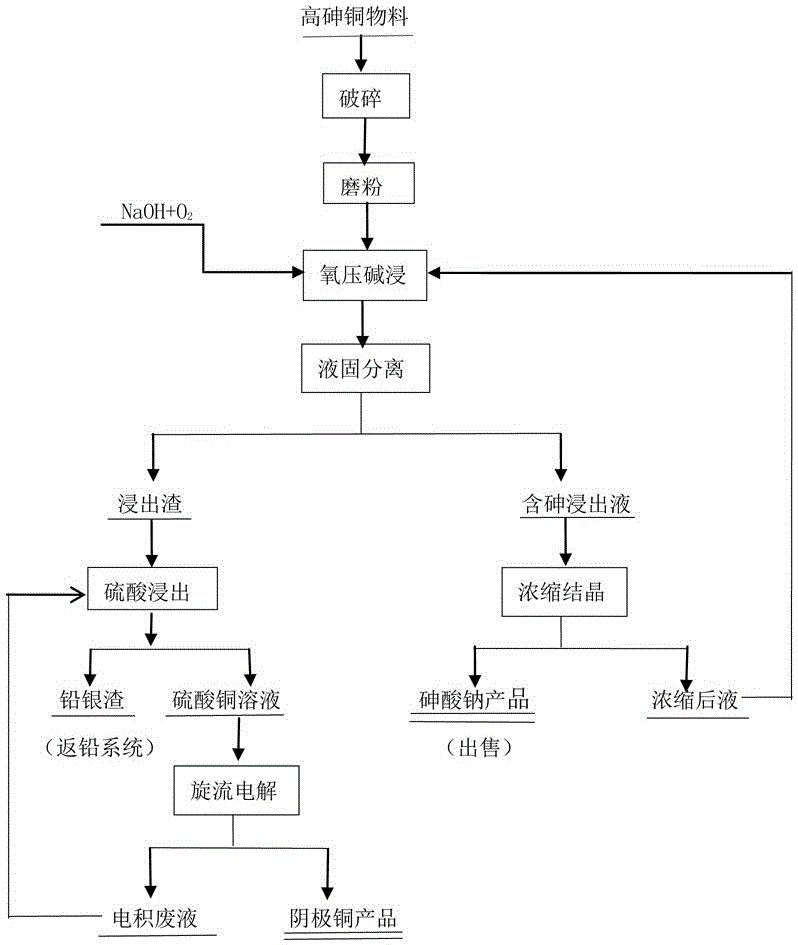
Technology for treating high-arsenic copper material
ActiveCN106555058AEnvironmental pollutionNo pollution to the environmentPhotography auxillary processesProcess efficiency improvementElectrolysisCopper sulfate
The invention discloses a technology for treating a high-arsenic copper material. The technology comprises the steps that the high-arsenic copper material is subjected to crushing, grinding, sodium hydroxide mixing and oxidizing leaching, copper in the high-arsenic copper material is oxidized and left in residues in a residual mode along with lead and noble metal of gold, silver and platinum, arsenic enters a solution in a sodium arsenate mode, leachate is subjected to concentration and crystallization, a sodium arsenate product is obtained, and a concentrated solution returns to be subject to oxygen-pressure alkali leaching; leached residues are subjected to sulfuric-acid atmospheric pressure leaching, copper enters the solution in a copper sulfate mode, acid adjustment is conducted, and vortex electrolysis is directly conducted for extracting copper; electrodeposited waste liquid is recycled; and lead and the noble metal enter lead and silver residues, and valuable elements of Pb, Ag and Au are comprehensively recycled. The technology belongs to a clean metallurgy process, is low in equipment corrosion-resistant requirement, free of pollution to environment, easy to operate and high in comprehensive recycling degree of metal and has the advantages of being higher in practicability and adaptability to raw materials and the like.
Owner:CHENZHOU CITY JINGUI SILVER IND CO LTD
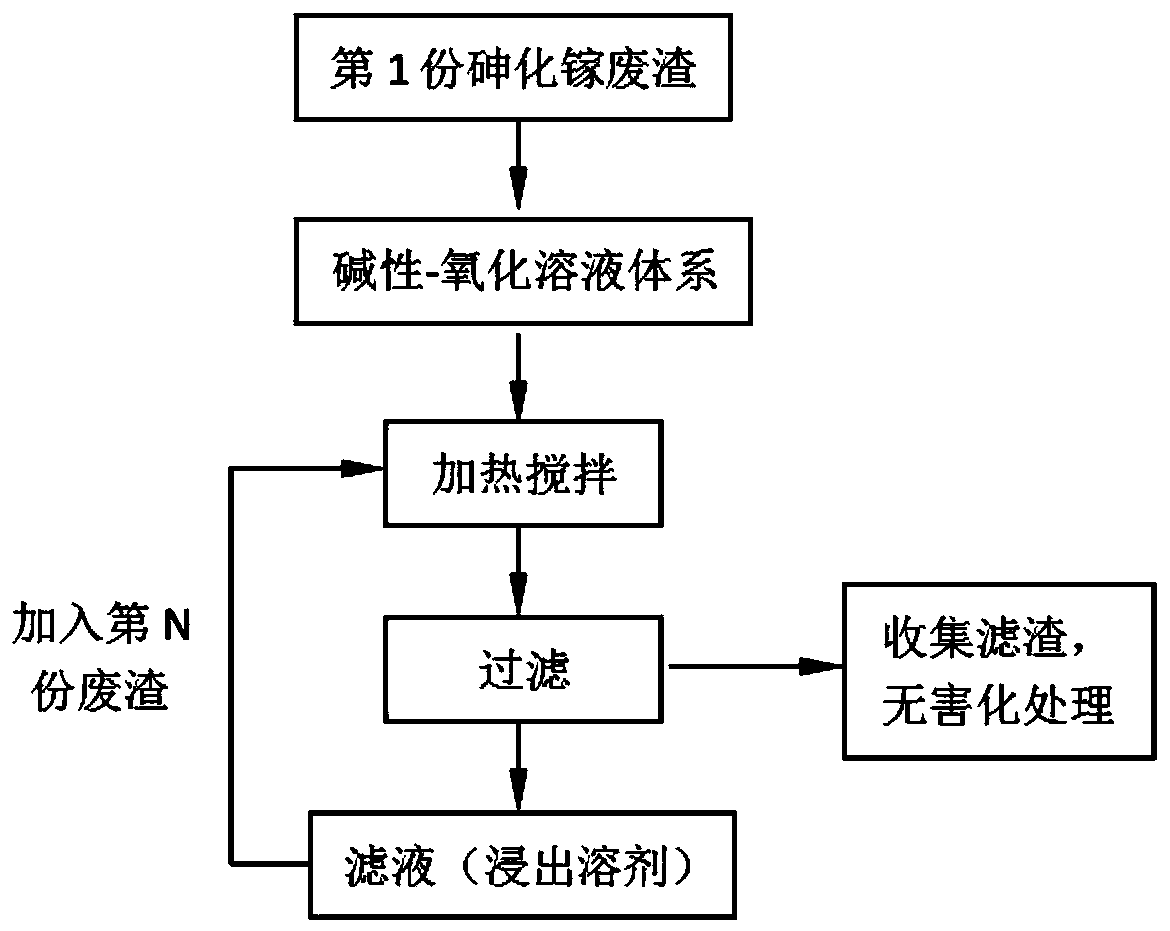
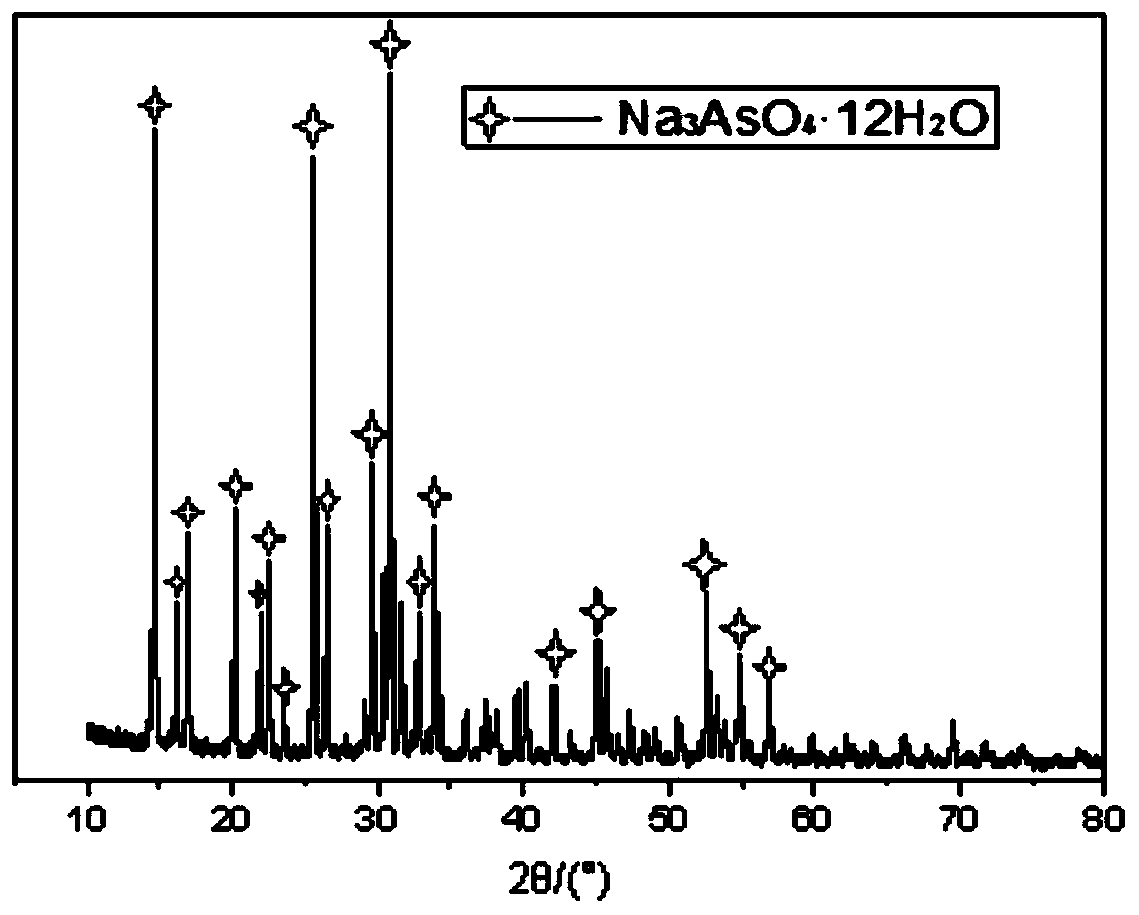
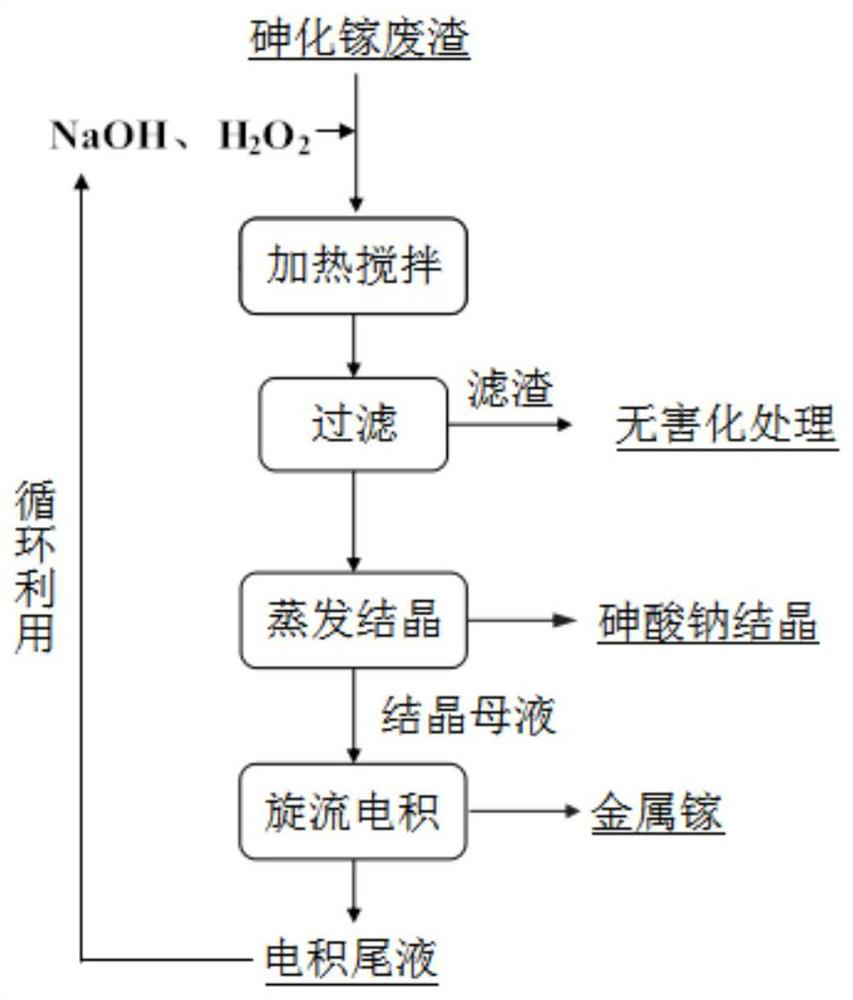
Method for recycling and preparing sodium arsenate and gallium metal from gallium arsenide waste residue
ActiveCN110938742AEasy to separateHigh purityPhotography auxillary processesArsenites/arsenatesGallium arsenateGallium
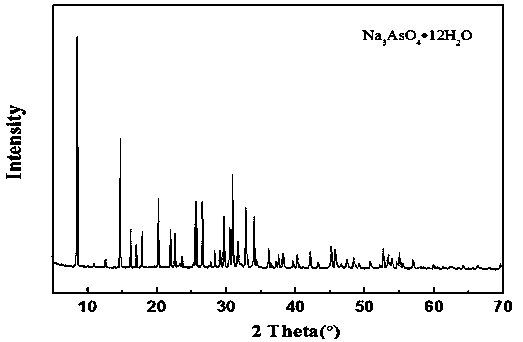
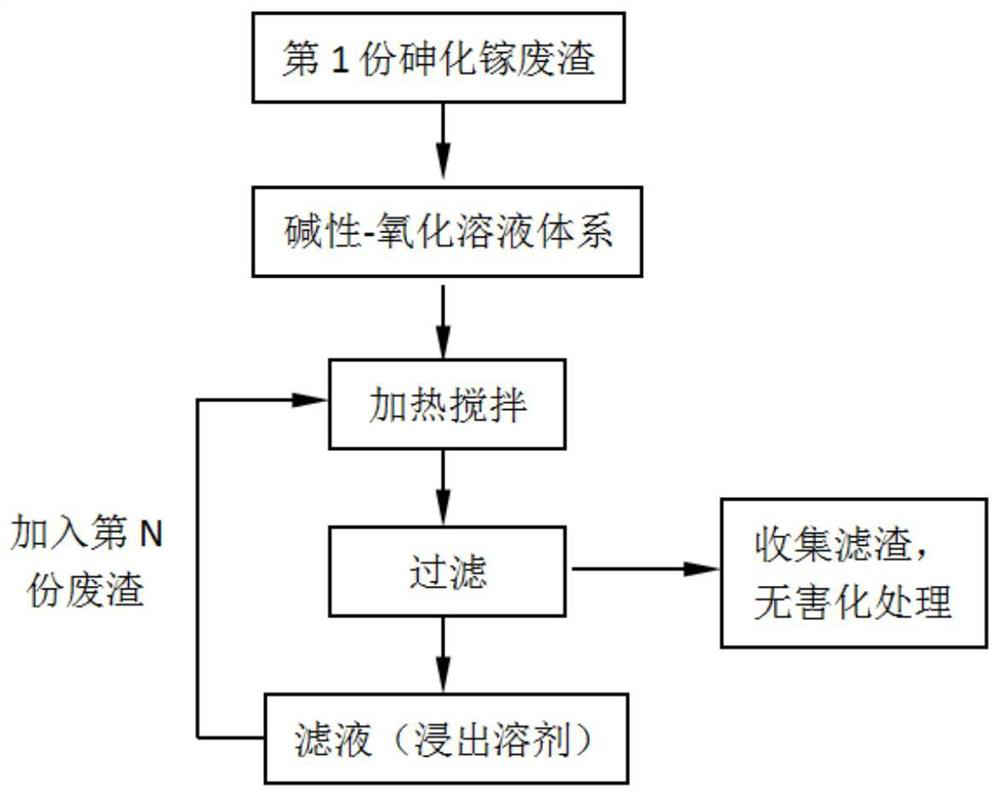
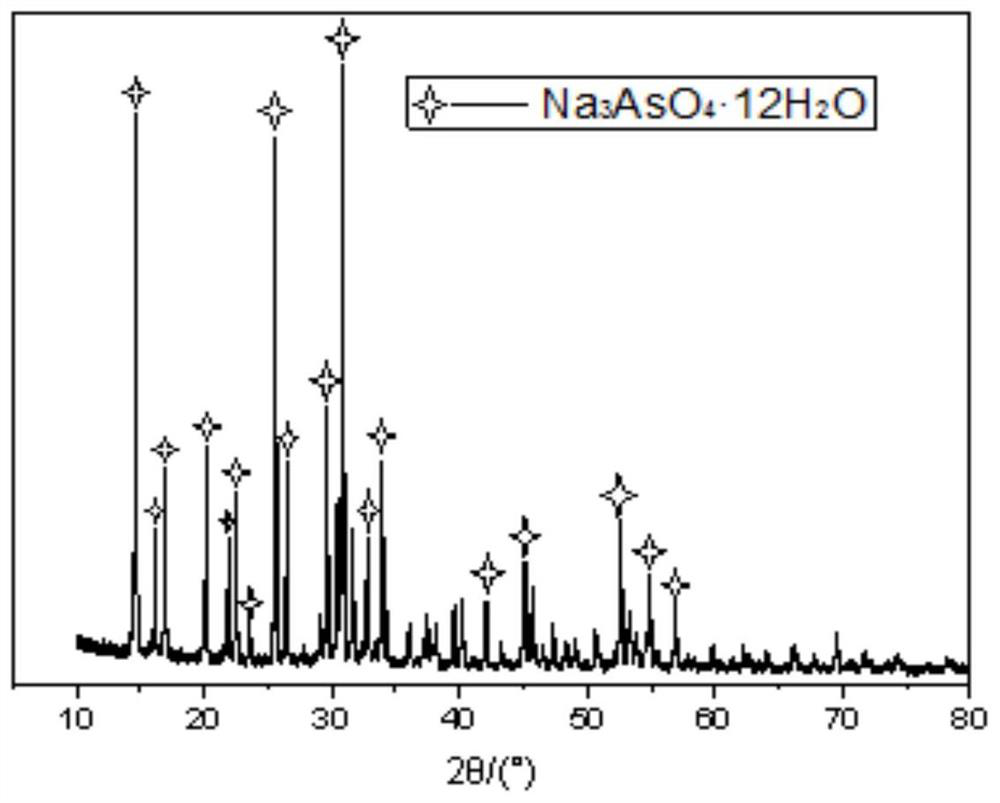
The invention relates to a method for recycling and preparing sodium arsenate and gallium metal from gallium arsenide waste residue. The method comprises the following steps of leaching the gallium arsenide waste residue in an alkaline-oxidized solution system after the gallium arsenide waste residue is dried, crushed and screened, wherein the conditions of the NaOH concentration, the oxidant concentration, the time, the temperature and the like during leaching are controlled so that arsenic and gallium are simultaneously quickly dissolved in a leaching solution to the great extent; performingevaporative crystallization to make the sodium arsenate crystallize preferentially to realize quick effective separation of the arsenic and the gallium; performing recrystallization to improve the purity of the sodium arsenate; and performing cyclone electrowinning to obtain high-purity gallium metal. The raw material gallium arsenide waste residue is from solid waste landfill and is sampled without cost; according to the method provided by the invention, the sodium arsenate and gallium metal are recycled and prepared from the waste residue; the purity of the prepared sodium arsenate is at least 95 percent; the purity of the prepared gallium metal is at least 3N; the prepared sodium arsenate and gallium metal have great utilization value; the recycled and purified sodium arsenate can serve as a chemical raw material; and the method disclosed by the invention effectively solves the problems of environment pollution and resource waste and meets the requirements of sustainable development society.
Owner:YANGZHOU NINGDA NOBLE METAL CO LTD
Comprehensive recovery method of smoke with high arsenic and antimony oxide contents
A comprehensive recovery method of smoke with high arsenic and antimony oxide contents comprises the following steps of: (1) roasting: after uniformly mixing the smoke with the high arsenic and antimony oxide contents, sodium nitrate and alkali, putting in a furnace for roasting at 400-680 DEG C, cooling after roasting to obtain a roasting material, (2) leaching: adding the roasting material into hot water for leaching, filtering to obtain a sodium antimonate filter cake and a sodium arsenate solution, and (3) drying: drying the sodium antimonate filter cake and the concentrated sodium arsenate solution to obtain a sodium antimonate product and a sodium arsenate product. The method integrates the advantages of a fire method and a wet method, and thoroughly separates antimony and arsenic from the smoke with the high arsenic and antimony oxide contents; an antimony content in sodium antimonate is 56-62%; an arsenic content in sodium antimonate is less than 0.5%; the arsenic content in sodium arsenate is 17-30%; the antimony content in sodium arsenate is less than 0.5%; the direct yield of the method reaches above 95%; recovery rates of antimony and arsenic are 96-99%; a recovery process is short; very little waste gas is generated and can be absorbed and returned by an alkaline liquor; and the method is short in flow and environment-friendly.
Owner:锡矿山闪星锑业有限责任公司
Process for comprehensively recovering high arsenic polymetallic material
ActiveCN102392136ALower requirementSimple operating conditionsProcess efficiency improvementArsenic pollutionPersulfate
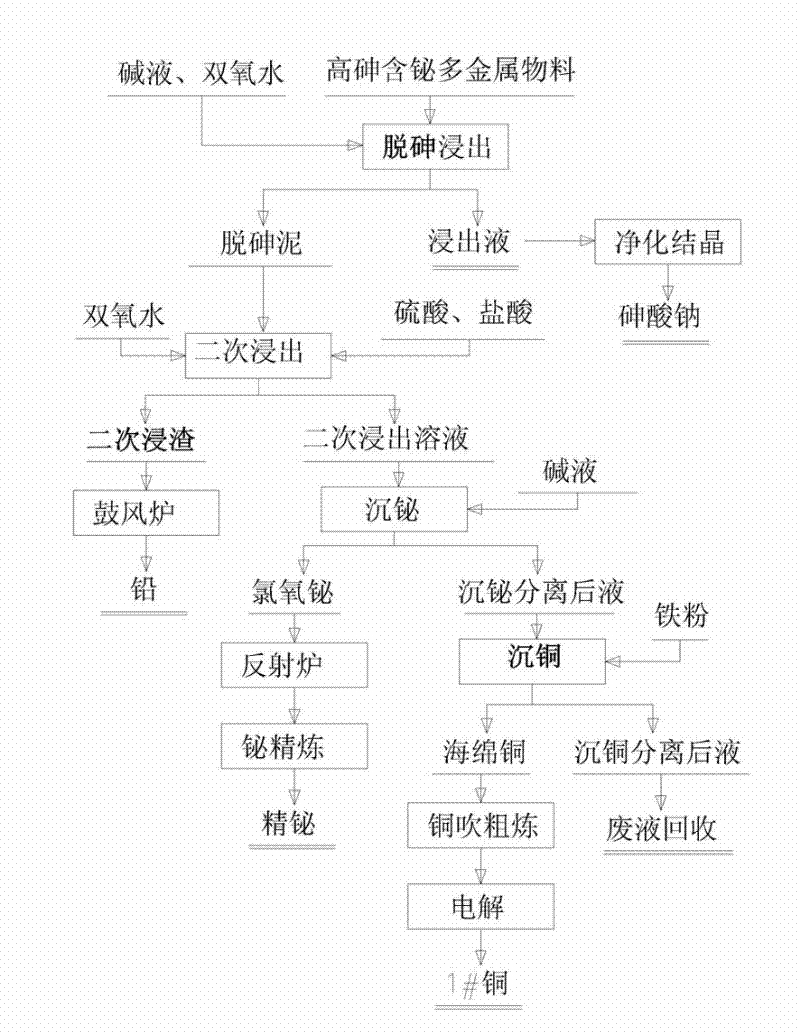
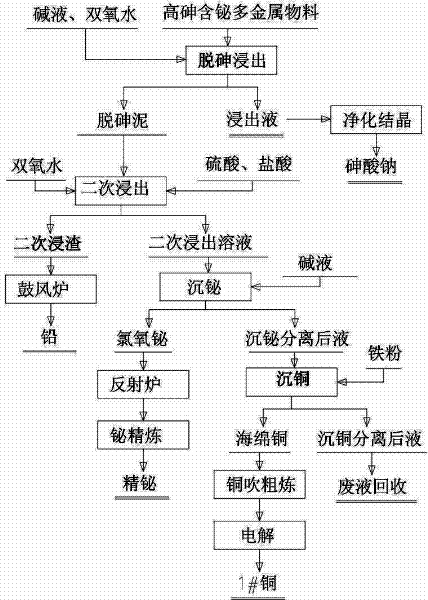
The invention relates to a process for comprehensively recovering a high arsenic polymetallic material, which belongs to the non ferrous metal comprehensive recovery field. The process comprises the following steps: leaching arsenic in the polymetallic material from the polymetallic material by sodium hydroxide, hydrogen peroxide and water, routine purifying a leachate and removing impurities, crystallizing to obtain sodium arsenate; leaching the arsenic removal mud after removing arsenic by sulfuric acid, hydrochloric acid and hydrogen peroxide twice, sending leaching residue to a blast furnace for refining lead; adding alkali loquor in a leaching solution for regulating pH value to 2.0-2.5, depositing bismuth by a bismuth chlorine oxygen form, separating and depositing bismuth chlorine oxygen and refining to obtain the refined bismuth; adding iron powder in a solution which is performed bismuth deposition and separation, electrolyzing to obtain 1#copper; sending solution which is performed copper deposition and separation to a waste liquid recovery pool for processing. The invention has the advantages of low production cost, simple operation condition and requirement, safe operation and no arsenic pollution. According to the invention, the arsenic recovery rate is greater than or equal to 96.5%, the lead recovery rate is greater than or equal to 98%, the bismuth recovery rate is greater than or equal to 95.8% and the copper recovery rate is greater than or equal to 96.5%.
Owner:郴州雄风环保科技有限公司
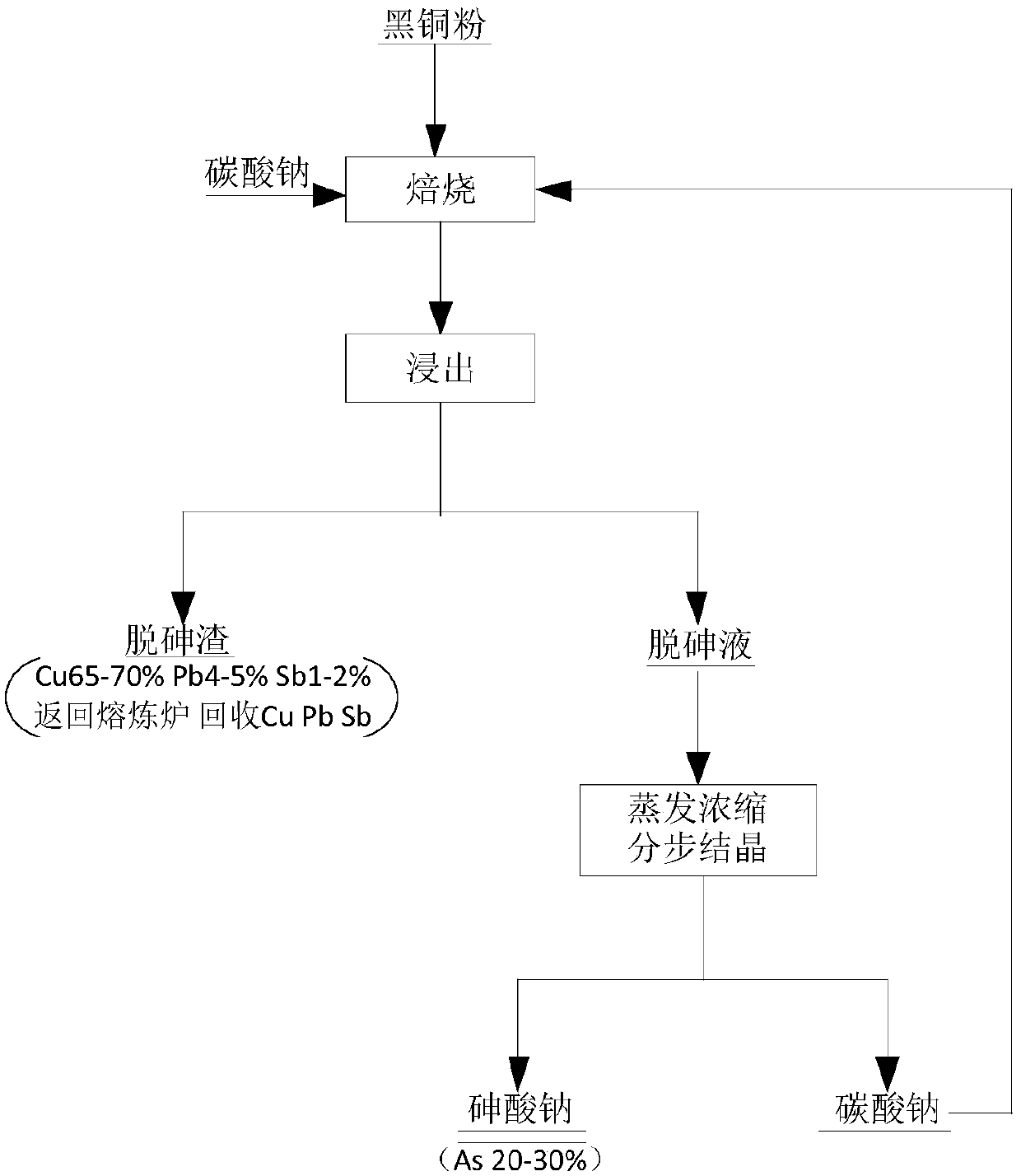
Method and application for removing arsenic from black copper sludge
InactiveCN108048664ALow impurity contentReduce pollutionArsenites/arsenatesProcess efficiency improvementEnvironmental resistanceSludge
The invention provides a method and application for removing arsenic from black copper sludge, and relates to the technical field of arsenic removal. The method for removing the arsenic from the blackcopper sludge comprises the steps that the black copper sludge and alkali are mixed and roasted; and after a roasted product and water are mixed, infiltrated and filtered, arsenic removal liquid is subjected to fractional crystallization to obtain a sodium arsenate product and alkali. The method is easy and convenient to operate and high in controllability, alkali can be cyclically utilized, resource regeneration is achieved, and environment protection and high efficiency are achieved.
Owner:GUANGDONG POLYTECHNIC OF ENVIRONMENTAL PROTECTION ENG
Pyrogenic copper smelting white smoke resource comprehensive utilization method
InactiveCN106834705AMeet the feed requirementsReduce manufacturing costArsenites/arsenatesProcess efficiency improvementElectrolysisLead smelting
The invention discloses a pyrogenic copper smelting white smoke resource comprehensive utilization method. The method comprises the following specific steps: white smoke and caustic soda are mixed in proportion; sodium sulfate is added, so that solution has a certain SO42- concentration; air is fed at 80-95 DEG C for oxidation leaching; then, the filtration is performed; and a proper amount of water is used for washing filter cakes. Filtrate has the main component of sodium arsenate, and is evaporated, condensed, cooled, crystallized, filtered and separated to obtain a sodium arsenate double salt product; and mother liquor after separation of sodium arsenate is returned to the white smoke for alkali leaching. The filter cakes in alkali leaching are pulped; sulfuric acid is added for acid leaching at 80-95 DEG C; then, the filtration is performed; the filter cakes have the main component of lead sulfate; and lead sulfate serves as a lead concentrate for lead smelting. Filtrate is copper sulfate solution; iron powder is added for replacement to obtain sponge copper; and the sponge copper is returned to a pyrogenic copper smelting system for refining as electrolyzed copper. The leaching rate of arsenic in the white smoke can reach above 95%; such precious metals as copper, lead, gold and silver are nearly not leached; the leaching rate of copper is higher than 98%; the content of lead in acid leaching slag is higher than 50%; and such precious metals as gold and silver are further enriched.
Owner:HENAN ZHONGYUAN GOLD SMELTERY
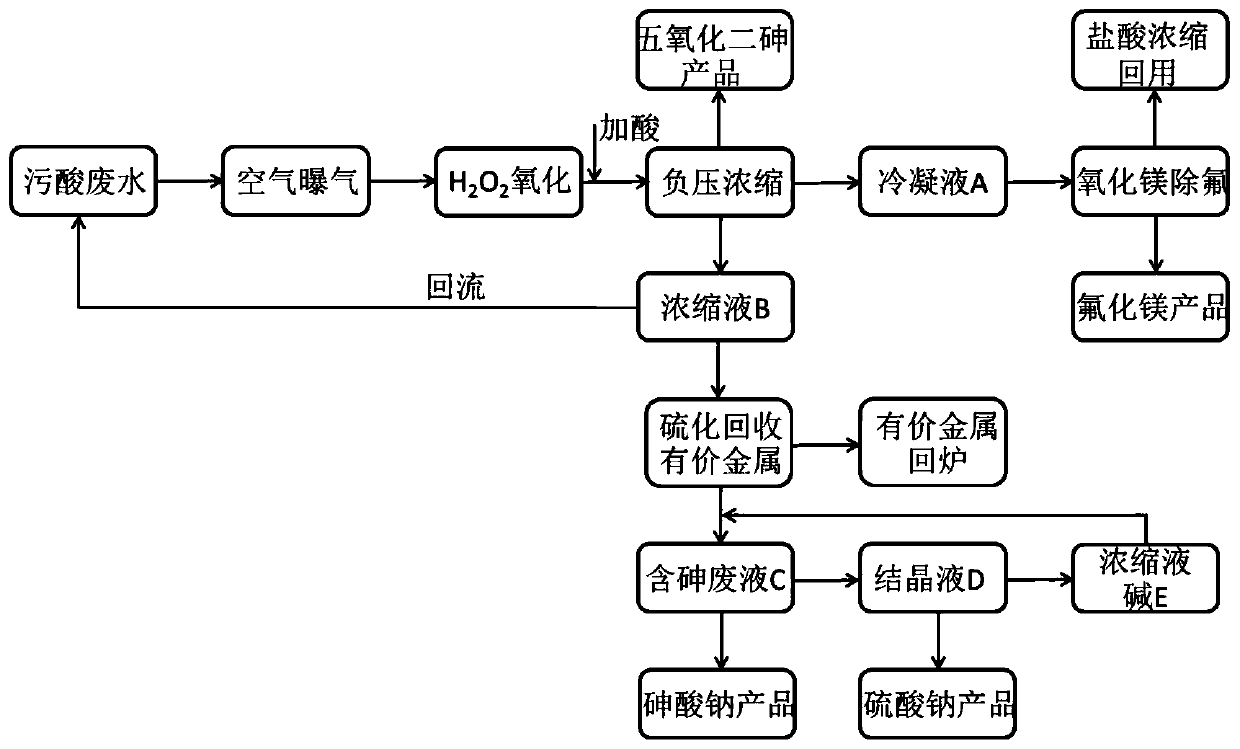
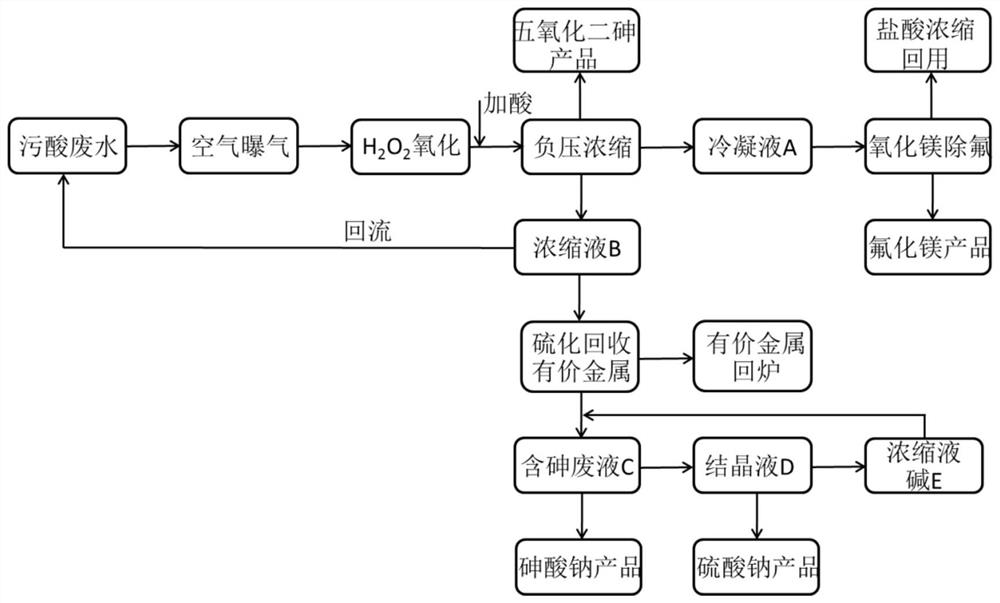
Method for resource utilization of waste sulfuric acid wastewater produced in copper smelting and acquisition of arsenic-containing products
ActiveCN111018229ANothing producedIncrease the areaMagnesium fluoridesChlorine/hydrogen-chloride purificationArsenic oxideArsenic pentoxide
The invention belongs to the technical field of industrial sewage treatment, and particularly discloses a method for resource utilization of waste sulfuric acid wastewater produced in copper smeltingand acquisition of arsenic-containing products. The method comprises the following steps: 1, oxidization of trivalent arsenic: oxidizing the waste sulfuric acid wastewater by air and hydrogen peroxidein sequence; 2, separation of arsenic from fluorine and chlorine: carrying out negative pressure evaporation after oxidation to obtain a condensate A containing fluorine and chlorine, a concentratedsolution B and arsenic pentoxide crystals; 3, separation of fluorine and chlorine: absorbing the condensate A with magnesium oxide to obtain a magnesium fluoride product and diluted hydrochloric acid;4, recovery of valuable metal: adding a sodium sulfide solution into the concentrated solution B to obtain a valuable metal precipitate and arsenic-containing waste liquid C, and returning the valuable metal precipitate to a furnace for refining; and 5, crystallization: adding caustic soda flakes or recycled concentrated alkali liquor E into the arsenic-containing waste liquor C, adjusting a pH value to 13.5-14.0 to obtain a sodium arsenate product and a crystallized filtrate D, filtering the crystallized filtrate D, and conducting concentrating to obtain a sodium sulfate product and the concentrated alkali liquor E. According to the method, waste sulfuric acid wastewater is subjected to resource recycling, and arsenic pentoxide and sodium arsenate products are obtained.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES +1
Method for separating arsenic and alkali in arsenic and alkali residues based on superfine ferric hydroxide colloid
ActiveCN108585057AReduce energy consumptionLow costIron oxides/hydroxidesAlkali metal carbonates shape formationSolubilityFerric hydroxide
The invention discloses a method for separating arsenic and alkali in arsenic and alkali residues based on a superfine ferric hydroxide colloid. The method includes the steps: leaching the arsenic andalkali residues by water, and performing antimony removal on leaching solution through oxidation to obtain solution containing sodium carbonate and sodium arsenate; adding iron arsenate crystal nucleus and the superfine ferric hydroxide colloid to perform reaction to obtain iron arsenate crystals, and performing solid-liquid separation to obtain solution containing sodium carbonate and an iron arsenate product; leading carbon dioxide into the solution containing the sodium carbonate to perform reaction to separate out sodium hydrogen carbonate crystals, and performing thermal decomposition onthe sodium hydrogen carbonate crystals to obtain a sodium carbonate product. According to the method, arsenate in arsenic and alkali residue leaching solution is adsorbed and converted by the superfine Fe (OH)3 colloid with high activity to generate stable iron arsenate precipitation with low solubility and good crystal property, arsenic and alkali in arsenic and alkali residue leaching solutionare effectively separated, and the method overcomes the shortcomings of low iron salt arsenic removal utilization efficiency, incomplete purification and the like under the strong alkaline condition,is simple in process and convenient to operate and meets industrial production.
Owner:CENT SOUTH UNIV
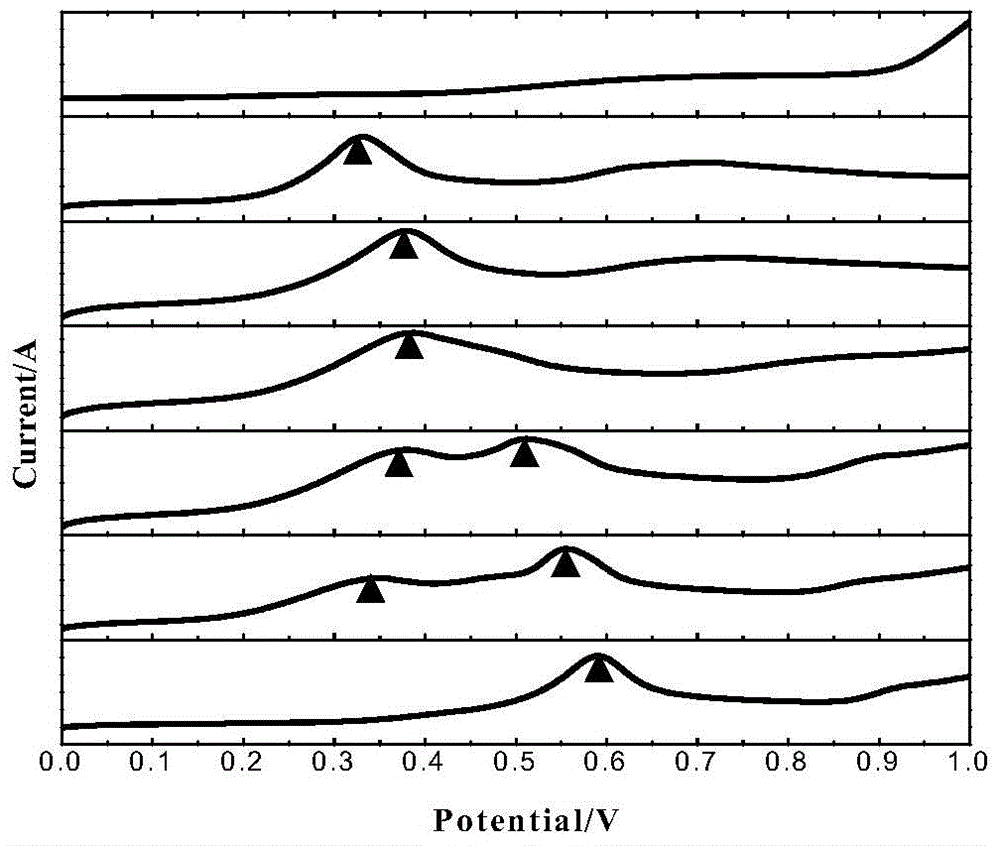
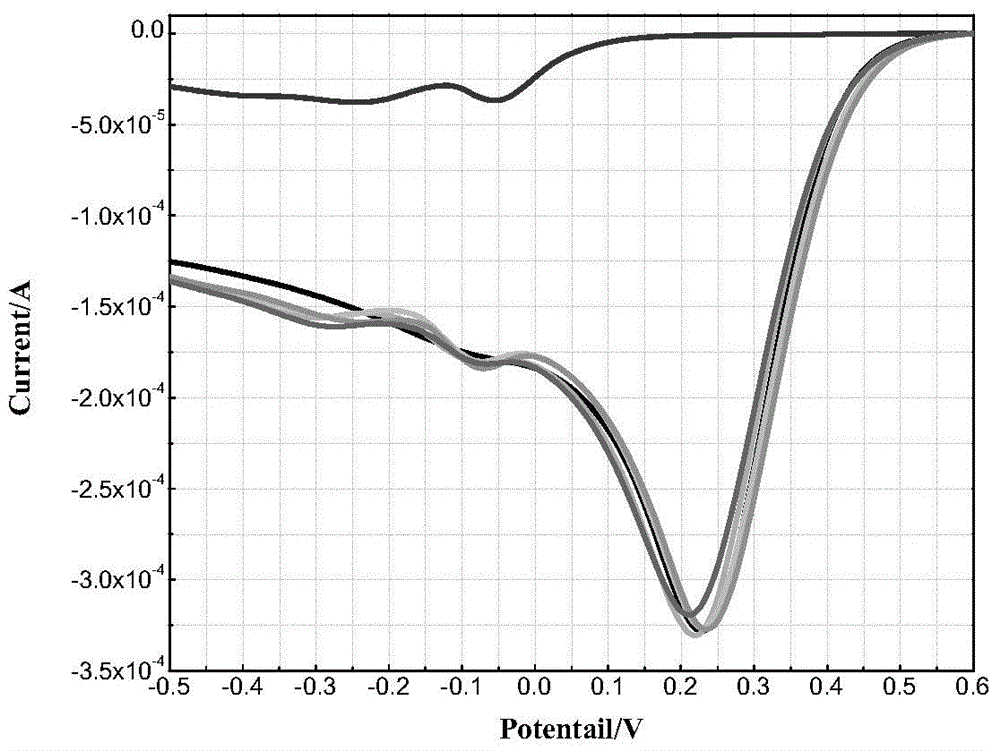
Method for assisted determination for unknown arsenic form in wastewater based on electrochemistry system
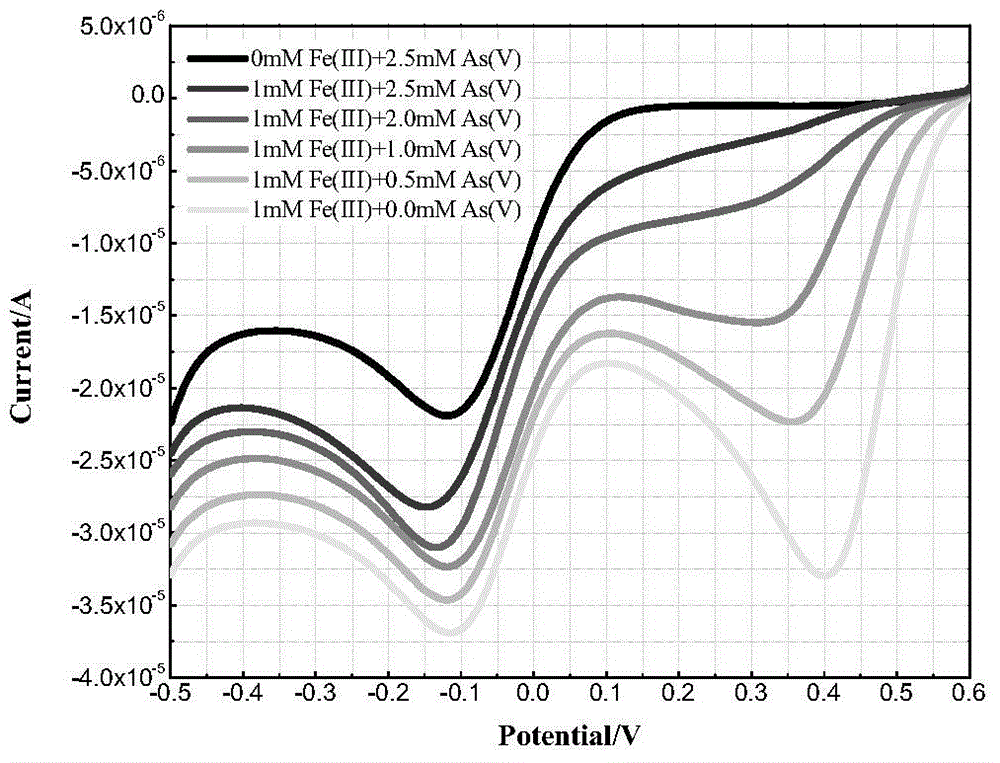
ActiveCN104407038ARealize in-situ real-time detectionSimple and fast operationMaterial electrochemical variablesSupporting electrolyteSodium arsenite
The invention discloses a method for assisted determination for an unknown arsenic form in wastewater based on an electrochemistry system. The method comprises the following steps: (1) establishing a three-electrode system: taking a gold electrode or a platinum electrode as a working electrode, taking a glassy carbon electrode as a counter electrode, and taking a saturated calomel electrode as a reference electrode; (2) preparing an experiment solution : using a sodium sulphate solution or a sulfuric acid solution as a supporting electrolyte to prepare the experiment solution, and using sodium arsenite, sodium arsenate, sodium carbonate and ferric sulfate to prepare an As (III)-CO32-(HCO3-) solution, an As (III)-Fe (III) solution and an As (V)-Fe (III) solution; (3) carrying out linear scanning detection. Based on the oxidation-reduction reaction and electrochemical principle of arsenic, the method adopts the linear scanning voltammetry of the three-electrode system to capture the change of a corresponding oxidation reduction potential when the arsenic form is changed, thereby predicting the unknown arsenic form in the wastewater containing arsenic. The method implements regulation and controlling for grasping the existing form of arsenic in the wastewater and provides methodology support for realization of deep purification.
Owner:CENT SOUTH UNIV
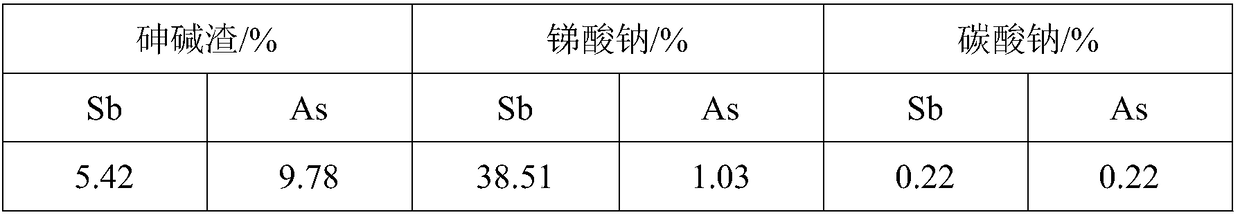
A method for separating and purifying sodium salt in arsenic-alkali slag
InactiveCN104818387BEfficient separation and recoveryLow As contentProcess efficiency improvementSodium saltImpurity
The invention aims at providing a method for separating and purifying sodium salts in arsenic alkali residue. The method can help separate high-purity sodium carbonate and sodium arsenate from antimony smelting arsenic alkali residue to achieve recycling of the sodium carbonate and sodium arsenate. The method comprises condensing a sodium arsenate composite salt solution at 60-70 DEG C to separate out most of Na2CO3 out of the solution, at the moment, all As is in the solution. Both magnetic fields and ultrasound can change the thermodynamic properties of chemical compounds and affect the solubility, the crystallization induction time, the width of the metastable region and the crystallization manner. A filter liquor a contains large amounts of Na+, under the coupling action of specific temperature and the magnetic field and ultrasound of the corresponding intensity, strong common-ion effects enable most of sodium arsenate to crystalize from the solution and other impurity components to stay in the solution. Meanwhile, by performing the pretreatment in the step 3) on the filter liquor a before the ultrasound and magnetic field treatment, the content of arsenic in crystalized sodium arsenate can be improved.
Owner:NANCHANG HANGKONG UNIVERSITY
Method for recovering metal from copper-smelting electrostatic precipitation ash and application
InactiveCN108048666AHigh purityLow impurity contentArsenites/arsenatesProcess efficiency improvementLead bismuthFiltration
The invention provides a method for recovering metal from copper-smelting electrostatic precipitation ash and an application and relates to the technical field of solid waste. The method comprises steps as follows: the copper-smelting electrostatic precipitation ash and an alkali are mixed and roasted; after a roasted product and water are subjected to mixing, infiltration and filtration, a filtrate and filtration residues are purified respectively, and products such as sodium arsenate, artificial lead-bismuth-silver ore, high-purity copper, zinc sulfate and the like are obtained finally. Themethod is easy and convenient to operate, high in controllability, high in comprehensive recovery rate and clean in process.
Owner:GUANGDONG POLYTECHNIC OF ENVIRONMENTAL PROTECTION ENG
Method for preparing sodium pyroantimonate and regenerating and recycling mother liquor
ActiveCN113371757AIncrease crystallization rateIncrease profitProcess efficiency improvementAntimonates/antimonitesZinc ionPhysical chemistry
The invention discloses a method for preparing a sodium pyroantimonate product from high-arsenic antimony white and vulcanizing, crystallizing, regenerating and recycling mother liquor, and relates to the field of antimony fine chemicals. According to the method, the low-cost-value high-arsenic antimony white is subjected to alkaline oxidation to prepare the sodium pyroantimonate product with the high additional value, the mother liquor containing impurities such as arsenic, lead and zinc is subjected to sulfuration crystallization to achieve cooling crystallization of sodium arsenate and sulfuration precipitation of lead and zinc ions, and the purified and regenerated mother liquor is continuously used for preparing the sodium pyroantimonate. The sodium pyroantimonate product meets the second-grade product requirement of the Chinese nonferrous metal industry standard (YS / T 22-2010), and the problem of separation of impurities such as antimony, arsenic, lead and zinc during preparation of sodium pyroantimonate from high-arsenic antimony white with complex components is solved. Meanwhile, the mother liquor is efficiently regenerated through vulcanization and crystallization, the problems that arsenic and alkali are difficult to separate and energy consumption is high after evaporation, concentration and crystallization are carried out are solved, and finally, the regenerated mother liquor is used for preparing sodium pyroantimonate, and efficient preparation of a product and recycling of the mother liquor are achieved; technical innovation is provided for high-valued utilization of low-price high-arsenic antimony white and purification and regeneration of mother liquor. The method has the advantages of convenience in operation, stable product components, high mother liquor purification efficiency and the like.
Owner:CENT SOUTH UNIV
Preparation method of hydroxyl arsenic ferrous phosphate solid solution and application of method
ActiveCN106115880AHigh crystallinityLong-term storage stabilityWater/sewage treatment by flocculation/precipitationEnvironmental resistanceIron sulfate
The invention discloses a preparation method of a hydroxyl arsenic ferrous phosphate solid solution and application of the method, and belongs to the technical field of environment protection. According to the method, iron sulfate heptahydrate, phosphoric acid and sodium arsenate or arsenic-containing waste water are respectively used as Fe sources, P sources and As sources; the reaction is performed for 6 to 72h at 100 to 200 DEG C; the hydroxyl arsenic ferrous phosphate solid solution with high crystallinity degree and high stability can be obtained through hydrothermal synthesis. The method is mainly used for converting pentavalent arsenic in a dissolved state in water into insoluble arsenic-containing precipitate compounds, so that the problem of environment pollution due to poor stability of the arsenic compounds can be solved. Compared with the prior art, the method has the advantages that the obtained arsenic-containing precipitate compounds have good long-time stability; the piling and the storage are safe and reliable; the secondary pollution of the arsenic is avoided.
Owner:KUNMING UNIV OF SCI & TECH
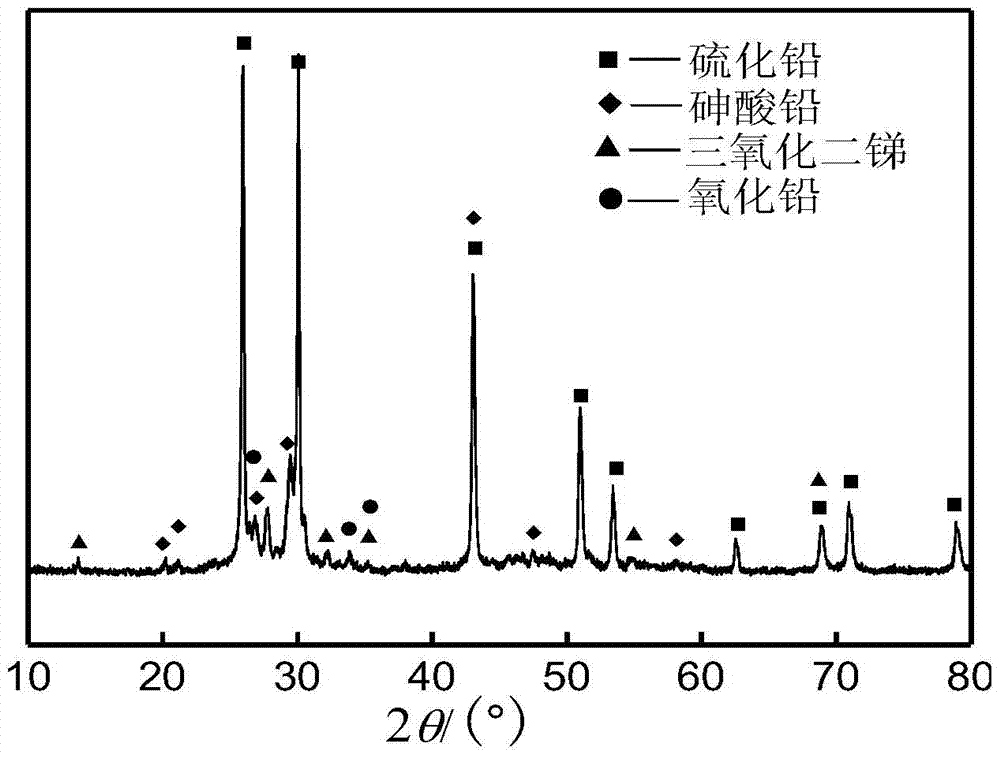
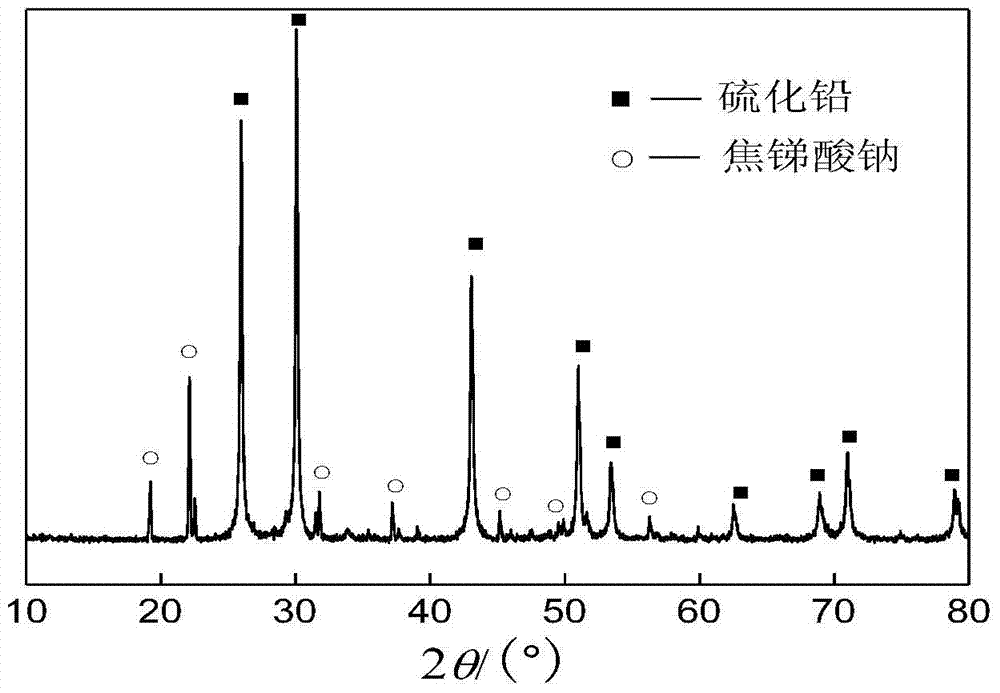
A method for preferentially removing arsenic from lead-antimony dust
ActiveCN105039722BHigh removal rateAchieve selective removalProcess efficiency improvementPregnant leach solutionRoom temperature
The invention discloses a method for preferably removing arsenic in lead and antimony smoke. The method comprises the following steps that firstly, sulphur and the lead and antimony smoke are mixed according to the mass ratio of 0.01-0.5:1, then the mixture is added into a sodium hydroxide solution to be leached, and meanwhile the temperature of the solution is kept within the range from 50 DEG C to 100 DEG C; secondly, liquid and solid separation is carried out on the solution leached in the first step, and a leaching agent and leaching residues are obtained; the leaching residues are washed, washing water obtained after washing is completed and the leaching agent are mixed and cooled to the room temperature, and filtering is carried out to obtain sodium arsenate crystal and crystalline liquid; and the crystalline liquid is used for leaching of the lead and antimony smoke in the first step. According to the technology method, the loss of valuable metal such as lead, antimony and zinc in the arsenic leaching and removing process is reduced through the adding of sulphur, meanwhile, the arsenic removing rate is increased, and selective removing of arsenic is achieved. The leaching rate of arsenic can reach 99% or more, and the content of arsenic in the leached residues can be reduced to 0.1% or lower.
Owner:CENT SOUTH UNIV
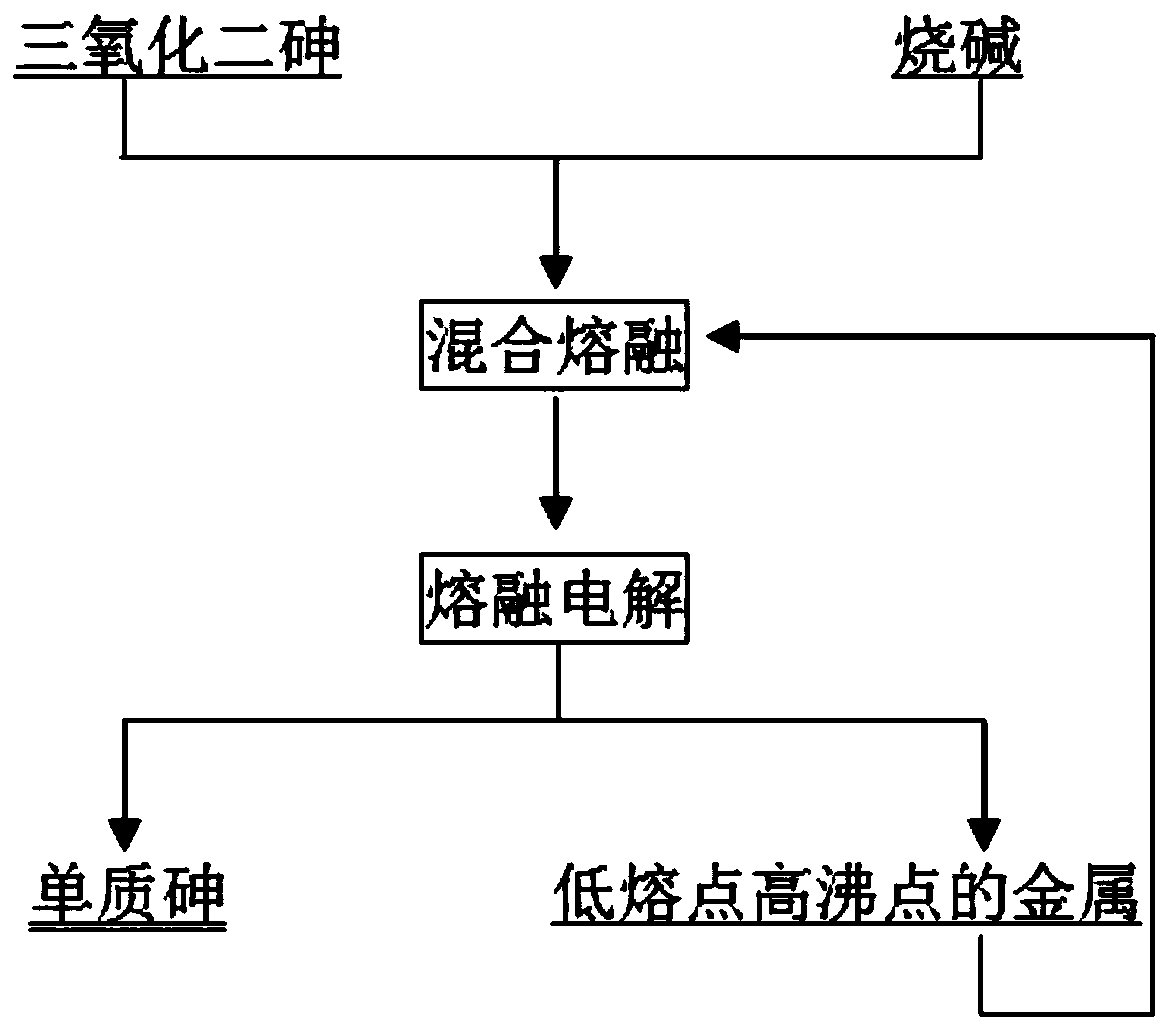
A kind of process of arsenic trioxide melting and electrolysis of elemental arsenic
The invention belongs to the fields of metallurgy technologies and environmental protection and relates to a technology for producing elementary arsenic from arsenic trioxide through fusion and electrolysis. The technology comprises the following steps: mixing and fusing the arsenic trioxide and caustic soda to obtain fused sodium arsenate, and carrying out electrolysis by using graphite as an electrolysis positive electrode and using metal with a low melting point and a high boiling point as an electrolysis negative electrode to obtain elementary arsenic and arsenic alloy with a low melting point; carrying out vacuum distillation on the arsenic alloy with the low melting point to obtain elementary arsenic and metal melt with a low melting point and a high boiling point; and collecting theelementary arsenic by a condenser, returning the metal melt with the low melting point and the high boiling point and continuously using the metal melt as the negative electrode. The technology can prevent the production of toxic arsenic hydride and at the same time prevent the arsenic from evaporating under the protection of a base covering agent, so that the direct recovery rate of the arsenicis greatly increased. The direct recovery rate of the arsenic is increased from traditional 60% to 95%, energy consumption is saved, and the production rate is increased.
Owner:湖南腾驰环保科技有限公司
Treatment method of arsenic-antimony smoke dust
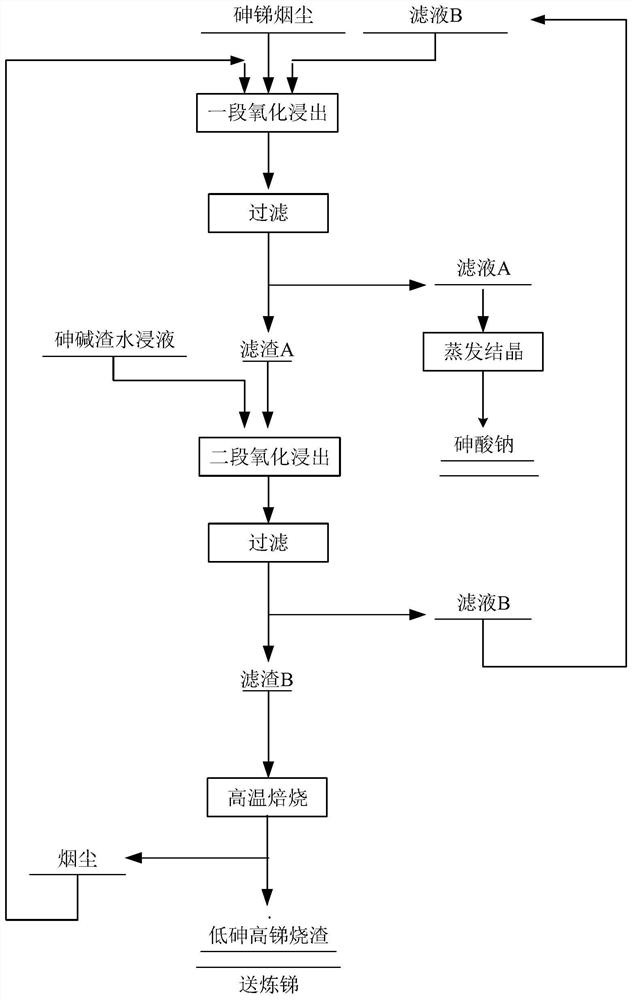
PendingCN113699380AAchieving zero emissionsLow costProcess efficiency improvementEnvironmental engineeringDisodium arsenate
The invention provides a treatment method of arsenic-antimony smoke dust. The treatment method comprises the following steps that the arsenic-antimony smoke dust is added into an alkaline leaching agent to be subjected to first-stage oxidation leaching, filtering is conducted after leaching, and filtrate A and filter residues A are obtained; the filter residues A are added into the alkaline leaching agent to be subjected to second-stage oxidation leaching, filtering is conducted after leaching is completed, and filtrate B and filter residues B are obtained; the filtrate A is subjected to evaporative crystallization, and sodium arsenate crystals are obtained; and the filter residues B are subjected to high-temperature roasting treatment, and low-arsenic high-antimony cinder is obtained. The treatment method disclosed by the invention is simple to operate, relatively complete separation of arsenic and antimony in the arsenic-antimony smoke dust and zero discharge of wastewater can be realized, high-valence alkali, hydrogen peroxide and other agents which are used in a large amount in a conventional process are saved, the treatment cost is greatly reduced, and the treatment method is suitable for large-scale popularization and application.
Owner:HUNAN RES INST FOR NONFERROUS METALS
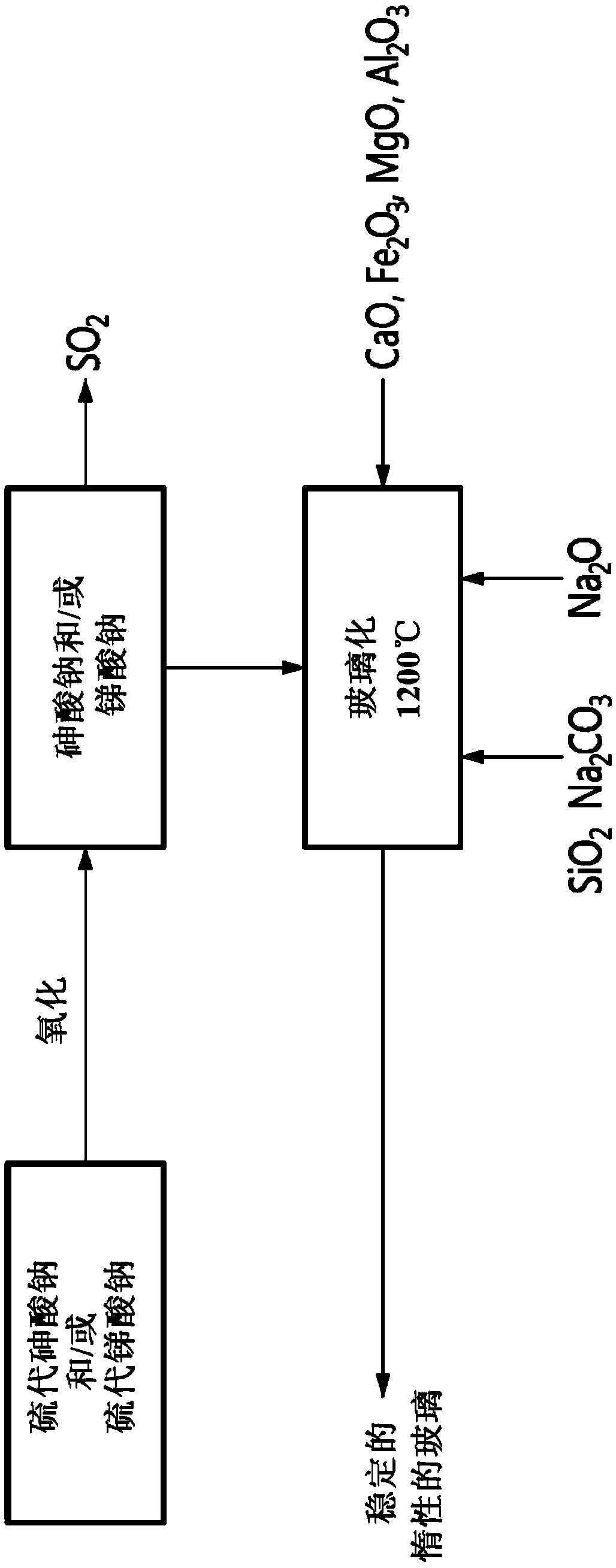
Method for vitrification of arsenic and antimony
A method for vitrification of arsenic and antimony, comprising substituting oxygen to sulfur on thiosalts, incorporating resulting sodium arsenate and sodium antimonate into a sodium silicate glass-forming mixture and vitrifying the sodium silicate glass-forming mixture into a resulting glass sequestering the arsenic and antimony.
Owner:DUNDEE SUSTAINABLE TECH
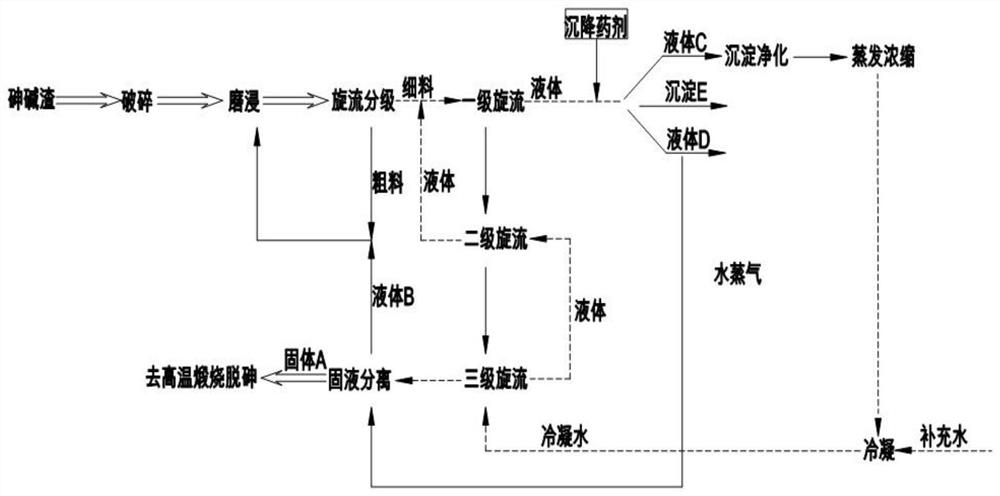
A system and method for synergistic treatment of arsenic-alkali slag grinding leaching dealkalization and cement kiln resource utilization
ActiveCN109776001BReduce consumptionReduce in quantityCement productionAlkali metal oxides/hydroxidesAlkali activated slagCo-processing
The invention discloses an arsenic-alkali slag grinding leaching dealkalization and cement kiln resource utilization cooperative treatment system and process. The arsenic-alkali slag is sequentially processed through crushing, grinding leaching dealkalization, lye purification and lye evaporation. The alkali process is washed many times, and the alkali content in the residue after dealkalization is greatly reduced. The sodium arsenate and sodium antimonate in the solution are converted into water-insoluble precipitation substances under the action of the precipitant, which is beneficial to high-temperature calcination and removal of arsenic. The arsenic and antimony in the alkali slag, the lye produced during the treatment process can be evaporated and concentrated, used as a smelting agent or desulfurizer, etc., the solid obtained after filtration can be calcined at high temperature, and the calcined residue can be used as cement raw materials. It saves production costs and meets the requirements of developing circular economy and sustainable development.
Owner:长沙中硅环保科技有限公司
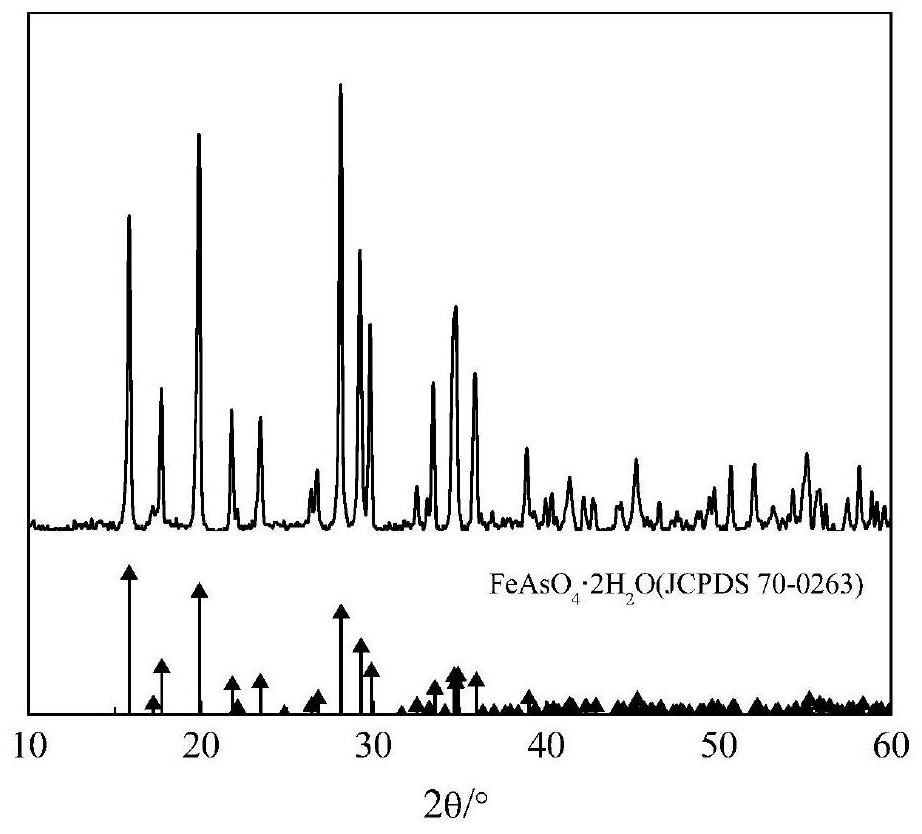
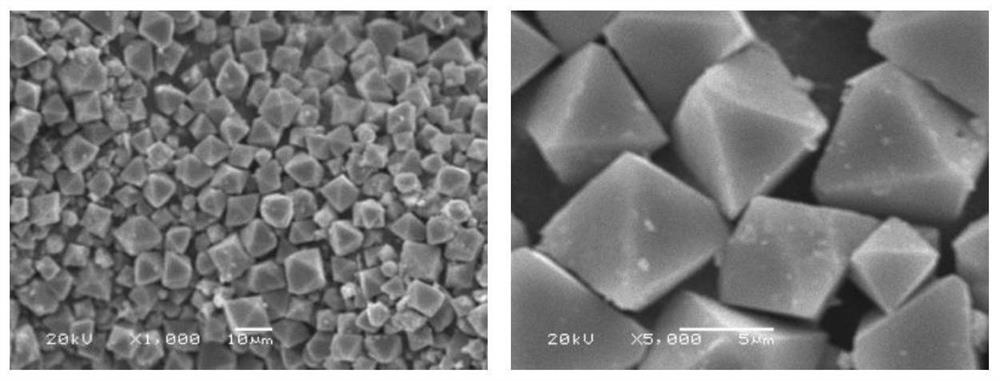
A method for fixing arsenic by stabilizing arsenic-alkali slag to prepare scorodite
ActiveCN109809494BRealize separation and purificationHigh purityIron compoundsAlkali metal carbonatesFerrous saltsSodium acid carbonate
The invention discloses an arsenic-fixing method for preparing scorodite by stabilizing the arsenic-alkali slag, which comprises the following steps: 1) oxidatively leaching the arsenic-alkali slag, and filtering to obtain a leaching solution containing sodium carbonate and sodium arsenate and sodium antimonate Precipitation; the leaching solution is concentrated and passed into CO 2 Dealkalization, filtering to obtain dealkalization leaching solution and sodium bicarbonate crystals; 2) adding acid to the dealkalization leaching solution obtained in step 1) to control its pH to 1.0-2.5 to obtain an arsenic-rich solution; 3) according to the iron-arsenic molar ratio of 1.0-3.0 To the arsenic solution obtained in step 2) add ferrous salt and H 2 o 2 Control the pH of the mixed solution to 1.2-2.0, and react at 75-95°C to obtain scorodite crystals. The present invention treats the arsenic-alkali slag to obtain scorodite crystals with biconical octahedral morphology and uniform particles, and the arsenic leaching concentration is lower than the provisions of GB5085.3-2007 "Identification Standards for Hazardous Wastes-Leach Toxicity Identification" and can be stored safely for a long time .
Owner:CENT SOUTH UNIV
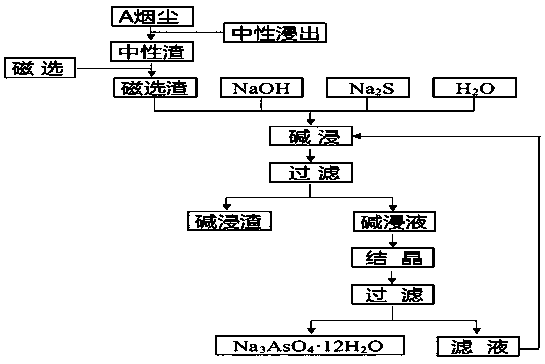
Method for preparing high-purity sodium arsenate from copper smelting smoke dust through alkaline leaching
InactiveCN111394583ATake advantage ofFully sustainableProcess efficiency improvementPhysical chemistryDisodium arsenate
The invention discloses a method for preparing high-purity sodium arsenate from copper smelting smoke dust through alkaline leaching. Copper smelting plant flash furnace smoke dust serves as a main raw material, after neutral leaching, solid-liquid separation is carried out, neutral leaching liquid and neutral leaching slag are obtained, the neutral leaching slag is subjected to wet process magnetic separation, then, solid-liquid separation is carried out, and magnetic separation slag is obtained; the magnetic separation slag is subjected to wet process alkaline leaching further, solid-liquidseparation is carried out, and alkaline leaching liquid is obtained; and the alkaline leaching is frozen, and high-purity sodium arsenate crystals can be obtained. The method is suitable for opening and arsenic extracting of the copper smelting smoke dust, enrichment and recycling of valuable elements in the smoke dust can be achieved, the process flow is clear and efficient, the resource use rateis high, and the method is economic, practical, safe, environment-friendly and good in industry prospect and social benefits.
Owner:FUZHOU UNIV
A method for recovering and preparing sodium arsenate and metal gallium from gallium arsenide waste slag
ActiveCN110938742BEasy to separateHigh purityArsenites/arsenatesPhotography auxillary processesGallium arsenatePhysical chemistry
The invention relates to a method for recovering and preparing sodium arsenate and metal gallium from gallium arsenide waste slag. The gallium arsenide waste slag is dried, crushed and sieved and then leached in an alkaline-oxidizing solution system to control the leaching time. NaOH concentration, oxidant concentration, time, temperature and other conditions, so that arsenic and gallium are rapidly dissolved in the leachate to the greatest extent at the same time, and then through evaporation and crystallization, sodium arsenate is preferentially crystallized to achieve rapid and effective separation of arsenic and gallium; Crystallization improves the purity of sodium arsenate; high-purity metal gallium is obtained by swirl electrowinning. The gallium arsenide waste slag, the raw material of the present invention, comes from solid waste landfills, and there is no cost for sampling. The method of the present invention recovers and prepares sodium arsenate and metal gallium from the waste slag. The purity of the prepared sodium arsenate is at least 95%, and the obtained The gallium metal has a purity of at least 3N and has high utilization value, and the recovered and purified sodium arsenate can be used as a chemical raw material; the method of the invention effectively solves the problems of environmental pollution and waste of resources, and meets the requirements of a sustainable development society.
Owner:YANGZHOU NINGDA NOBLE METAL CO LTD
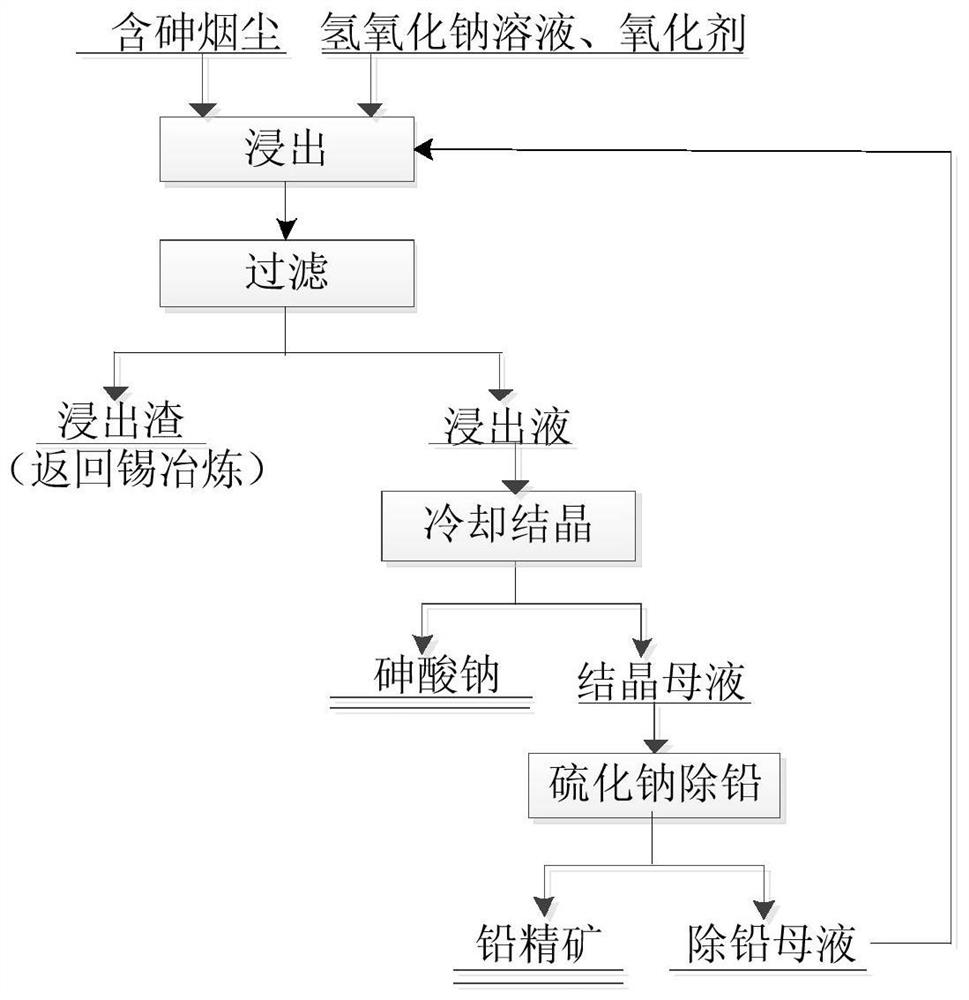
A method for removing arsenic from arsenic dust
ActiveCN110257624BAchieve separationNo emissionsArsenites/arsenatesProcess efficiency improvementPregnant leach solutionEnvironmental engineering
A method for removing arsenic from arsenic-containing fume, wherein the arsenic-containing fume contains 10-20% of arsenic, 20-30% of tin, 3% of indium, and 5-8% of lead; Steps such as permanent leaching, cooling and crystallization of the leachate, recovery of lead from the crystallization mother liquor, etc. The invention can effectively separate the arsenic from the tin and indium in the arsenic-containing dust, and can also produce the sodium arsenate product at the same time.
Owner:云南锡业研究院有限公司
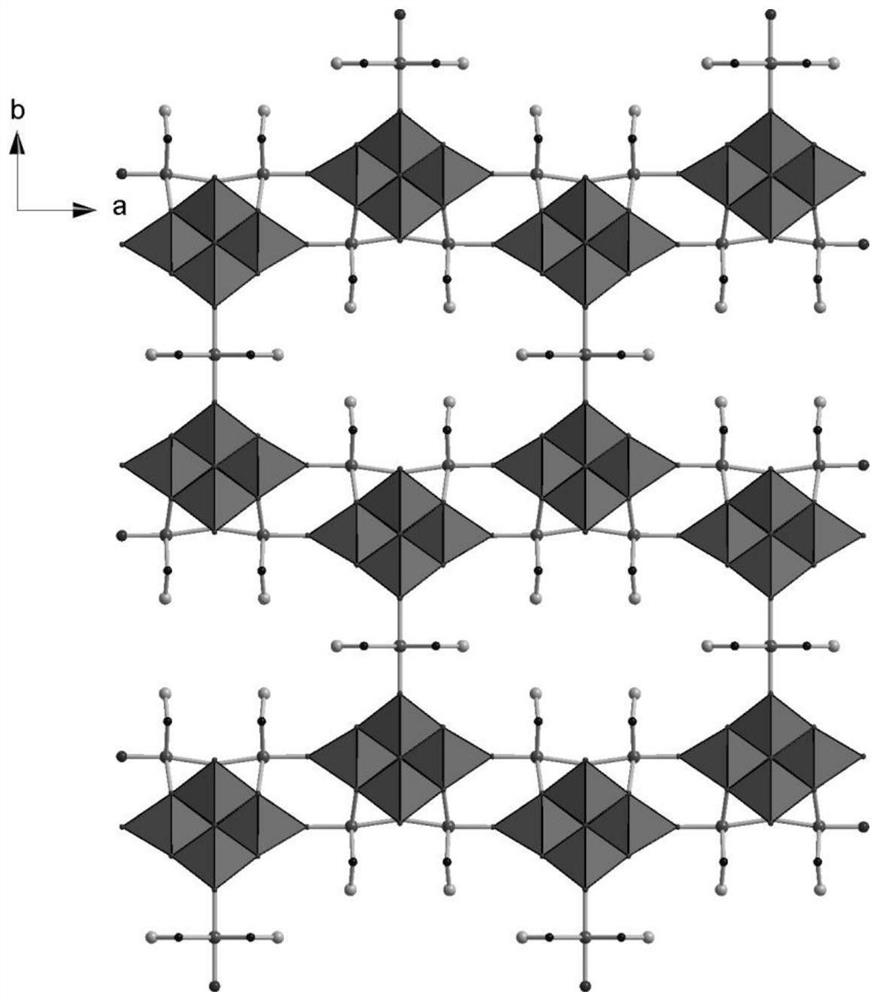
Cobalt niobium oxygen cluster and preparation method and application thereof
PendingCN114849781AExhibit photocatalytic oxygen production performanceLow priceOrganic-compounds/hydrides/coordination-complexes catalystsIron group organic compounds without C-metal linkagesHydration reactionEthylene diamine
The invention discloses a cobalt niobium oxygen cluster and a preparation method and application thereof, the cobalt niobium oxygen cluster is an organic-inorganic hybrid cobalt niobium oxygen cluster crystalline material, and the chemical formula of the cobalt niobium oxygen cluster crystalline material is Na3 [Nb6O19 {Co (en)} 2Co0.5 (en)]. 14H2O, the preparation method comprises the following steps: taking 0.458 g of K7HNb6O19. 13H2O (0.33 mmol), and dissolving the K7HNb6O19. 13H2O in 8 mL of water; in addition, 0.848 g of sodium arsenate Na3AsO4, 0.352 g of NaCl, 0.246 g of cobalt trichloride hexahydrate CoCl3. 6H2O and 1.4 mL of ethidene diamine are taken and added into the K7HNb6O19. 13H2O aqueous solution; stirring for 2.5 hours, transferring into a polytetrafluoroethylene reaction kettle, and sleeving with a shell; reacting at 150 DEG C for 4 days, and then cooling to room temperature to obtain a light red blocky crystal, namely Na3 [Nb6O19 {Co (en)} 2Co0.5 (en)]. 14H2O.
Owner:NANTONG UNIVERSITY
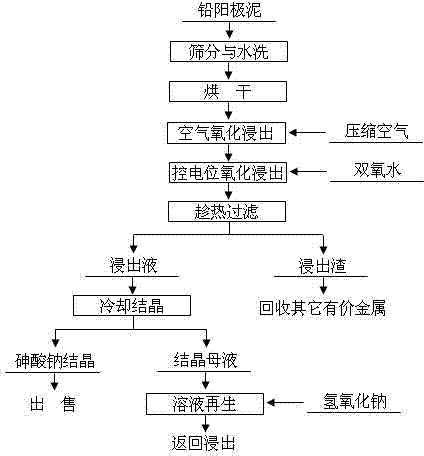
Method for removing and recovering arsenic from lead anode slime
ActiveCN101928838BHigh removal rateAvoid secondary pollutionProcess efficiency improvementArsenic pollutionSodium hydroxide
Owner:YONGZHOU FUJIA NON FERROUS METALS
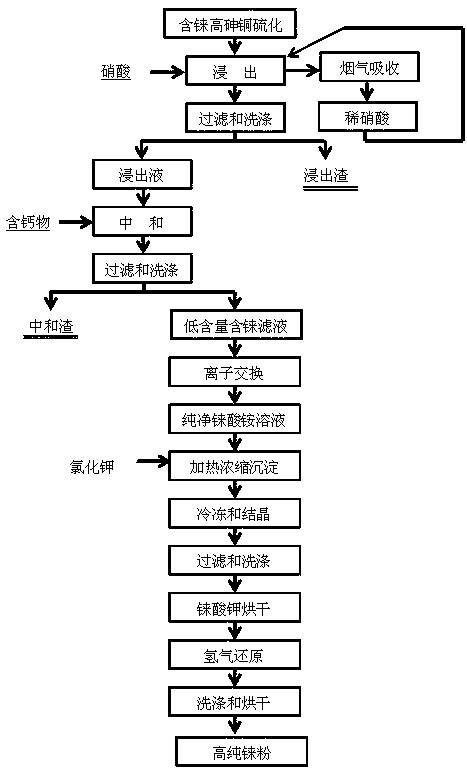
A method for preparing high-purity rhenium powder from rhenium-containing high-arsenic copper sulfide
The invention discloses a method for high-effectively enriching rhenium from rhenium-containing high-arsenic copper sulfide. The method includes: rhenium-rich slag and a certain amount of alkali are mixed to obtain size, and pressing, heating and alkaline leaching is performed, and after filtering and washing is performed, rhenium enters extract in the form of ammonium rhenate, and a part of arsenic enters the extract in the form of sodium arsenate. Reactive metal power is heated to replace the rhenium in the extract, and after filtering and washing is performed, the rhenium oxide is enriched, and separation between the rhenium and the arsenic can be achieved. The raw material of the method is the rhenium-rich slag which is obtained by sulfide precipitation of acidic wastewater generated during a production process of copper smelting enterprises and is rich in copper and arsenic; the method can fully leach the rhenium, and the metal power is used to replace the extract to obtain a rhenium-enrichment; the process of rhenium extraction can be simplified, and the cost of rhenium extraction is reduced. The method is simple in process, is short in flow, is high in enrichment ratio, is high in material adaptability, is low in cost, is easy to industrialize, has good application value, and can increase output value and profit of the copper smelting enterprises.
Owner:KUNMING METALLURGY COLLEGE
A resource comprehensive utilization method for purifying cobalt and nickel slag with zinc arsenic salt in hydrometallurgy
ActiveCN111575491BGuaranteed gradePrevent the occurrenceProcess efficiency improvementPregnant leach solutionSodium chlorate
The invention discloses a resource comprehensive utilization method for purifying cobalt-nickel slag by wet-method zinc-arsenic salt purification. Alkaline leaching is performed on the cobalt-nickel slag purified by wet-method zinc-arsenic salt, and alkali leaching solution and alkali leaching residue are obtained by filtration; The immersion solution is cooled and crystallized to obtain sodium arsenate. Dissolve the sodium arsenate with water and adjust the pH to 2-4, and then introduce SO 2 Reduction to obtain sodium arsenite solution; the alkali leaching residue is subjected to two-stage leaching, the first stage is low-acid leaching, and the second stage is high-acid leaching; copper powder is added to the low-acid leaching solution, copper powder is used to remove arsenic, and filtered to obtain The arsenic removal solution and the arsenic removal slag; the arsenic removal solution is subjected to controlled potential copper precipitation, and the copper precipitation solution and copper powder are obtained by filtering; the copper removal solution is added with sodium hypochlorite to oxidize and deposit cobalt. The recovery of the cobalt-nickel slag in the invention has the advantages of reasonable process, low separation cost, environmental protection and the like.
Owner:湖南株冶有色金属有限公司
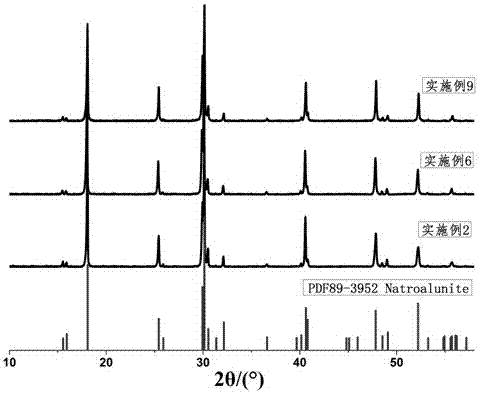
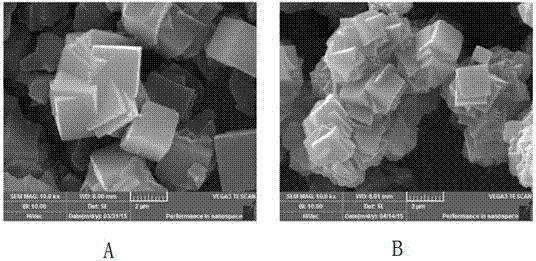
Method and application of low-temperature solid-phase synthesis of sodium arsenic alunite solid solution
The invention discloses a method for preparing arsenic-containing sulfate minerals and its application, in particular to a method for low-temperature solid-phase synthesis of sodium arsenic alunite solid solution and its application in the treatment of arsenic-containing waste residue. The method uses aluminum sulfate Or aluminum nitrate is the Al source, sodium sulfate is the Na source, sodium arsenate, sodium arsenite or arsenic-containing waste residue is the As source, and the crystallinity is high and the stability Good sodium arsenite solid solution; this method is mainly used to transform the easily soluble arsenic substance in the solid phase into insoluble sodium arsenite solid solution, so as to solve the high pressure conditions and reaction conditions required for the traditional hydrothermal synthesis of arsenite. The disadvantages of the kettle are high requirements, high energy consumption, high economic cost, and environmental pollution caused by the poor stability of traditional arsenic compounds; compared with traditional technologies, the obtained arsenic sodium alumite solid solution has good long-term stability, safe and reliable storage, and low economic cost , The reaction conditions are close to industrialization.
Owner:KUNMING UNIV OF SCI & TECH
A method for resource utilization of copper smelting sulfuric acid polluted acid wastewater and obtaining arsenic-containing products
ActiveCN111018229BIncrease the areaMeet industrial quality requirementsMagnesium fluoridesChlorine/hydrogen-chloride purificationArsenic oxideEngineering
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com