A resource comprehensive utilization method for purifying cobalt and nickel slag with zinc arsenic salt in hydrometallurgy
A technology for hydrometallurgical smelting of zinc and cobalt-nickel slag, applied in the direction of improving process efficiency, can solve problems such as wastewater discharge, achieve comprehensive recovery, eliminate the risk of arsenic metal pollutants, and reduce production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
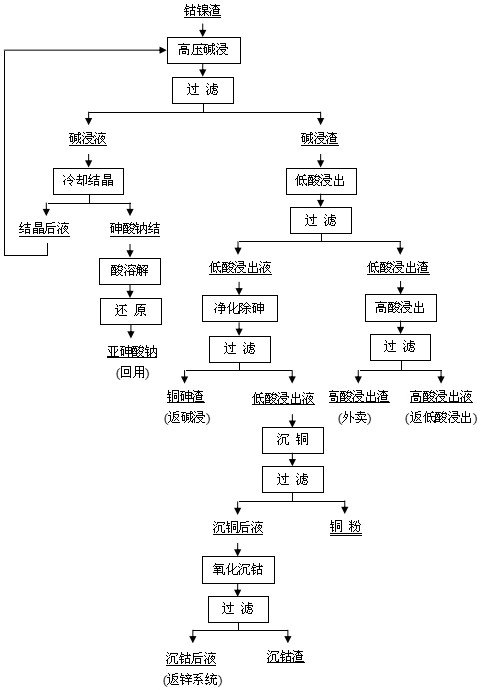
[0033] A. High-pressure alkaline leaching of arsenic: the mass percentage concentration is: Zn12.37%, Cu55.45%, Co3.41%, As13.28%, Pb3.24%, Ag0.017% cobalt-nickel slag, in alkali The leaching is carried out under the condition of a concentration of 5.2mol / L, the leaching temperature is 125°C, the liquid-solid ratio is 4:1, pure oxygen with an oxygen partial pressure of 2.6MPa is introduced, the leaching time is 3h, and the alkali leaching solution and alkali leaching solution are obtained by filtration scum.
[0034] B. Arsenic reduction: Cool and crystallize the alkali immersion solution obtained in step A to obtain sodium arsenate, dissolve the sodium arsenate crystals with water to obtain a sodium arsenate solution, adjust the pH of the sodium arsenate solution to 2, and then press SO 2 The molar ratio of arsenic to sodium arsenate solution is 2.05:1 and SO is passed through 2 , carry out the reduction reaction, the reduction time is 3.0h, and obtain the sodium arsenite so...
Embodiment 2
[0041] A. High-pressure alkaline arsenic immersion: the mass percent concentration is: Zn 12.37%, Cu 55.45%, Co 3.41%, As13.28%, Pb 3.24%, Ag 0.017% cobalt-nickel slag, at an alkali concentration of 3.8mol / L, the leaching temperature is 130°C, the liquid-solid ratio is 4:1, the oxygen partial pressure is 3.0MPa pure oxygen, the leaching time is 5h, and the alkali leaching solution and alkali leaching residue are obtained by filtration.
[0042] B. Arsenic reduction: Cool and crystallize the alkali immersion solution obtained in step A to obtain sodium arsenate, dissolve the sodium arsenate crystals with water and adjust the pH to 4, then press SO 2 The molar ratio to the arsenic in the sodium arsenate solution is 2.15:1 and the SO 2 , carry out the reduction reaction, the reduction time is 1.5h, and the sodium arsenite solution is obtained, and the saturated diarsenic trioxide crystals precipitated during the reduction process are dissolved with sodium hydroxide solution into...
Embodiment 3
[0049] A. High-pressure alkaline arsenic immersion: the mass percentage concentration is: Zn 12.37%, Cu 55.45%, Co 3.41%, As13.28%, Pb 3.24%, Ag 0.017% cobalt-nickel slag, at an alkali concentration of 4.8mol / L, the leaching temperature is 128°C, the liquid-solid ratio is 5:1, pure oxygen with an oxygen partial pressure of 2.8MPa is introduced, the leaching time is 4h, and the alkali leaching solution and alkali leaching residue are obtained by filtration.
[0050] B. Arsenic reduction: Cool and crystallize the alkaline immersion solution obtained in step A to obtain sodium arsenate, dissolve the sodium arsenate crystals with water and adjust the pH to 3, and then introduce SO 2 Reduction to obtain sodium arsenite solution. Among them, the reduction time is 2.0h, SO 2 Dosage is SO 2 The molar ratio to arsenic is 2.10: 1, the arsenic trioxide crystal saturated and precipitated in the reduction process is dissolved with sodium hydroxide solution into a sodium arsenite solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com