Method for assisted determination for unknown arsenic form in wastewater based on electrochemistry system
An electrochemical and three-electrode system technology, applied in the field of detection, can solve the problems of high cost, stay in the prediction stage of complex ions, and cannot real-time in-situ detection, etc., and achieve the effect of simple operation and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Prepare 1mol / L NaAsO with deionized water 2 solution, 1mol / L Na 2 CO 3 solution and 0.1mol / L Na 2 SO 4 solution. With 0.1mol / L Na 2 SO 4 The solution is a supporting electrolyte, and the preparation series As(III)+CO 3 2- (HCO 3 - ) experimental solution, As(III) and CO in the experimental solution 3 2- The concentration is (1+0), (1+1), (1+2), (1+4), (1+8), (1+16), (0+16)mmol / L, and hydrogen Sodium oxide and sulfuric acid were used to adjust the pH of the solution to 10.00.
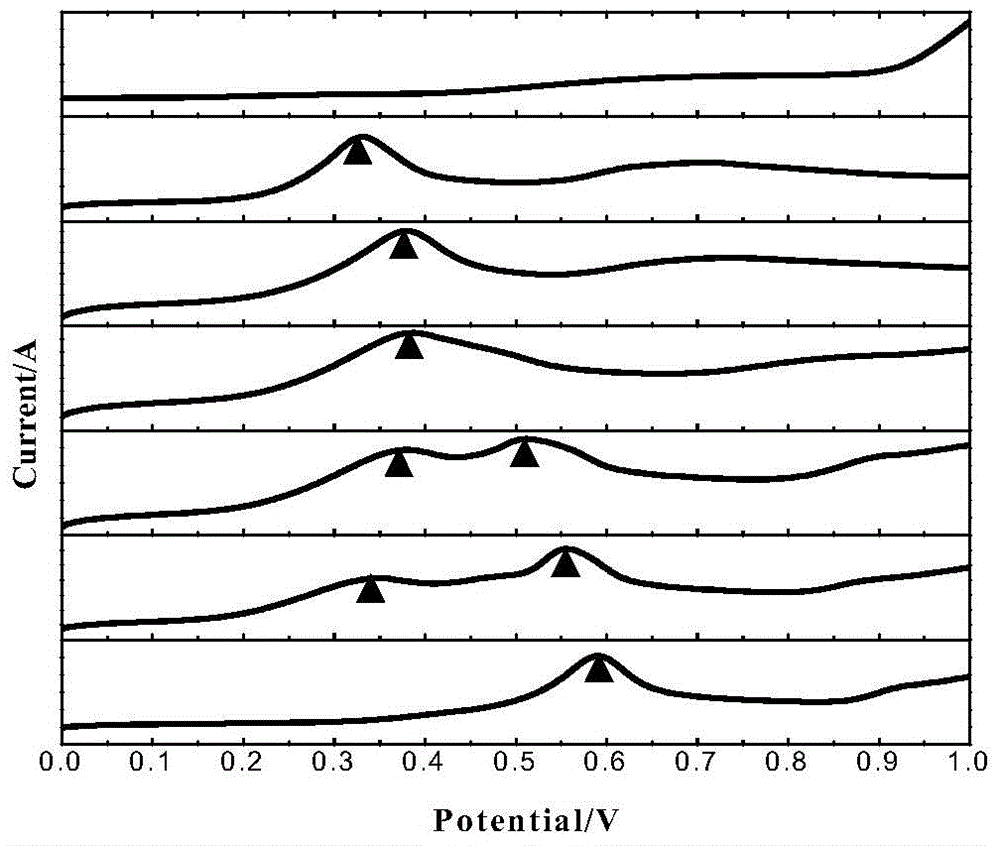
[0034] Each experimental solution was magnetically stirred at a speed of 500rpm for 30min, with a gold electrode as the working electrode, a glassy carbon electrode as the counter electrode, and a saturated calomel electrode as the reference electrode, and scanned from 0.2V forward voltammetry to 1.0V, the scanning speed Both are 50mV / s. A total of 7 curves were obtained, see the results figure 1 .
[0035] Such as figure 1 Shown: There is an obvious oxidation peak near 0.6V on th...
Embodiment 2
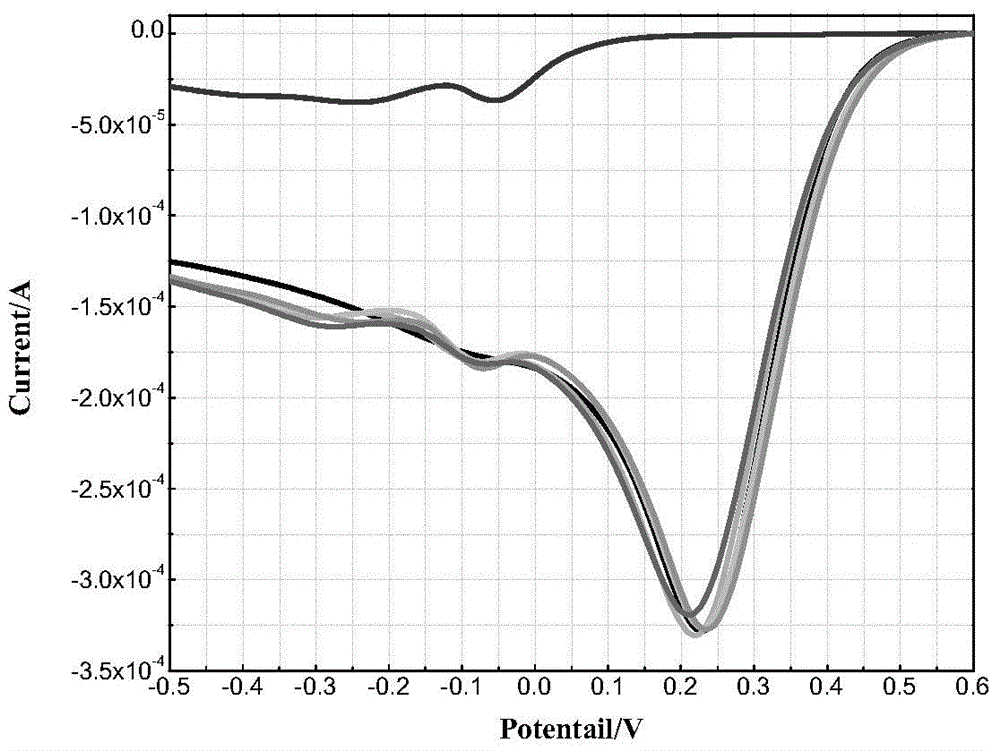
[0037] Prepare 1mol / L NaAsO with deionized water 2 solution, 5mmol / L of H 2 SO 4 solution. Prepare 0.05mol / L Fe with dilute sulfuric acid with a pH value of 2 2 (SO 4 ) 3 Solution, namely 100mmol / L Fe(III) solution. With 5mmol / L H 2 SO 4 The solution is used as a supporting electrolyte, and a series of As(III)+Fe(III) experimental solutions are prepared. The concentrations of As(III) and Fe(III) in the experimental solutions are (0+10), (5+10), (10+10 ), (20+10), (25+10), (25+0)mmol / L. Adjust the pH to 2 with sodium hydroxide and sulfuric acid.
[0038] Each solution was magnetically stirred at a speed of 500rpm for 30min, with a gold electrode as the working electrode, a glassy carbon electrode as the counter electrode, and a saturated calomel electrode as the reference electrode. Both are 50mV / s. A total of six curves were obtained, the results are shown in figure 2 , the experimental solution corresponding to the uppermost curve is a 25mmol As(III) solution wit...
Embodiment 3
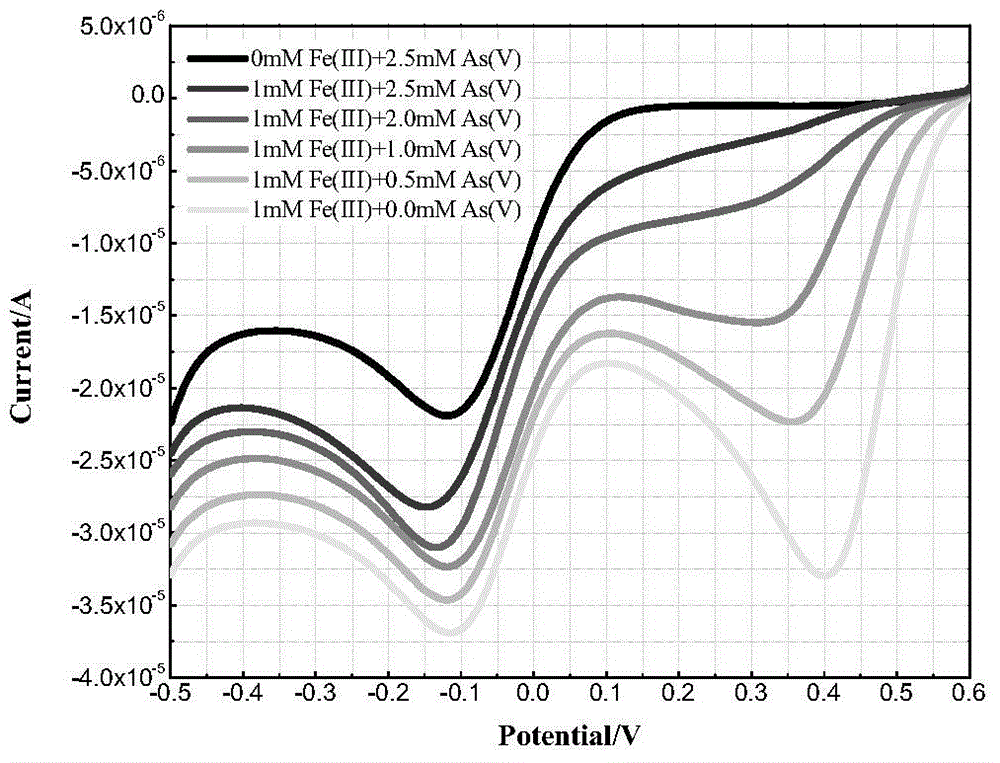
[0041] Prepare 0.1mol / L Na with deionized water 3 AsO 4 solution, 5mmol / L of H 2 SO 4 solution. Prepare 0.05mol / L Fe with dilute sulfuric acid with a pH value of 2 2 (SO 4 ) 3 Solution, namely 100mmol / L Fe(III) solution. With 5mmol / L H 2 SO 4 The solution is used as a supporting electrolyte, and a series of As(V)+Fe(III) experimental solutions are prepared. The concentrations of As(V) and Fe(III) in the experimental solutions are (0+1), (0.5+1), (1+1 ), (2+1), (2.5+1), (2.5+0)mmol / L, adjust the pH value to 2 with sodium hydroxide and sulfuric acid.
[0042] Each solution was magnetically stirred at a speed of 500rpm for 30min, with a gold electrode as the working electrode, a glassy carbon electrode as the counter electrode, and a saturated calomel electrode as the reference electrode. Both are 50mV / s. see results image 3 , the experimental solution corresponding to the uppermost curve is 2.5mmol / L As(V) solution without Fe(III), and the lower ones are (2.5+1), (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com