Photochromic perfluorocyclopentene molecular fluorescent probe compound and its preparation method and application
A perfluorocyclopentene, fluorescent probe technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., to achieve high selectivity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
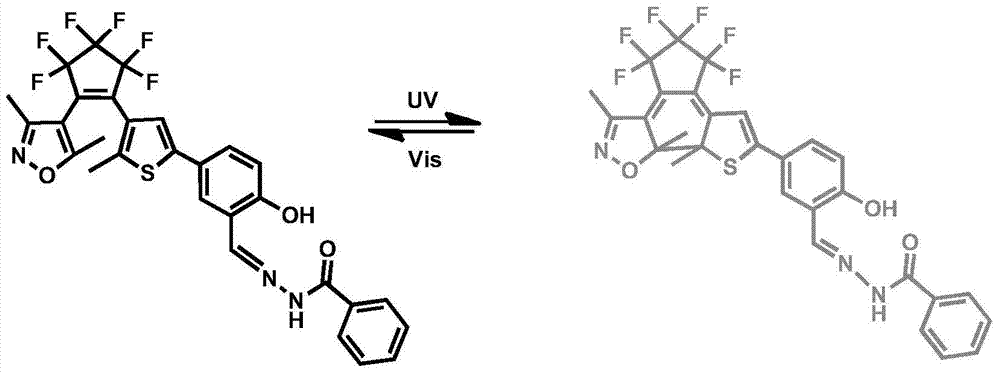
[0024] In the molecular structural formula, the final compound of the present invention is named as: {1-(3,5-dimethyl-4-isoxazolyl), 2-{2-methyl-5-[3-(methylene -Benzohydrazide)-4-hydroxyphenyl]-3-thienyl}} perfluorocyclopentene (1o), the synthetic scheme of this novel perfluorocyclopentene diarylethene photochromic compound is as follows Scheme 1 shows:
[0025] Scheme 1:
[0026]
[0027] Concrete synthetic steps are as follows:
[0028] 1. 1-[3,5-Dimethyl-4-isoxazole] perfluorocyclopentene (2)
[0029] Under the protection of argon and a liquid nitrogen acetone bath, 4-iodo-3,5-dimethylisoxazole (1) (1.12 g, 5 mmol) was dissolved in 50 mL of refined tetrahydrofuran. Inject n-butyllithium (5mmol, 2.3mL) under rapid stirring, continue the low-temperature reaction for 0.5 hours; inject perfluorocyclopentene (5mmol, 0.7mL) rapidly under high-speed stirring, continue the low-temperature reaction for 1.0 hour and then naturally rise to room temperature, and then add an app...
Embodiment 2
[0049] Example 2: The functional experiments of the compounds of the present invention are mainly reflected in two aspects: detection of metal ions as fluorescent probes and dual regulation of fluorescent molecular switches.
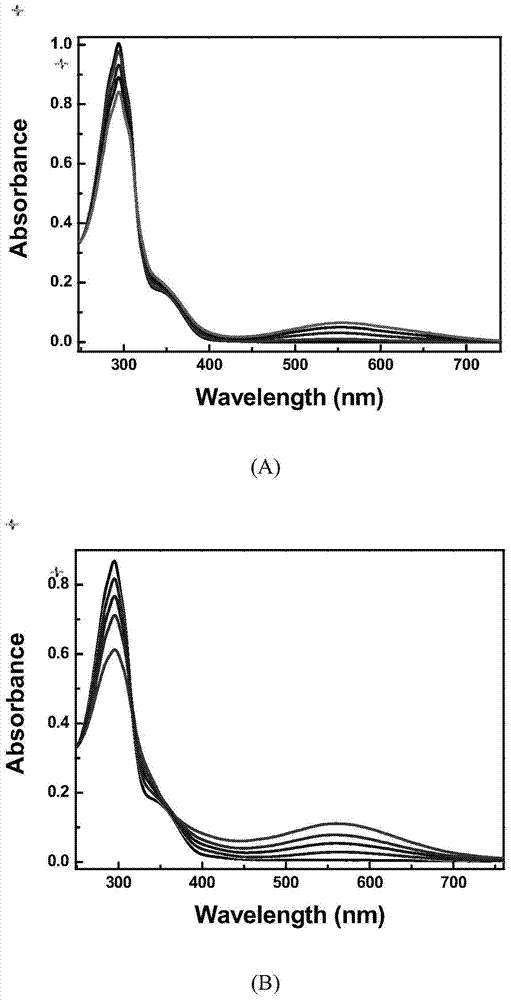
[0050] 1. Compound 1 detects metal ions in different solvent systems
[0051] Such as Figure 5 As shown in (A), in the acetonitrile solution of compound 1 (2.0×10 -5 mol L -1 ), after various metal ions were added in 3-fold equivalent, the fluorescence spectrum under 438nm light excitation changed significantly. in Pb 2+ ,Cu 2+ ,Al 3+ ,Cr 3+ ,Hg 2+ , Ni 2+ ,Mg 2+ ,Cd 2+ ,Co 3+ ,Mn 2+ , Ba 2+ ,Sr 2+ ,K + and Ca 2+ The fluorescence of the solution did not change significantly after the addition of the plasma. And only after adding Zn 2+ Afterwards, the solution had an obvious fluorescence emission peak at 560 nm, and the fluorescence intensity was enhanced by 200 times compared with the initial value. Due to Zn 2+ and Cd 2+ Most of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com