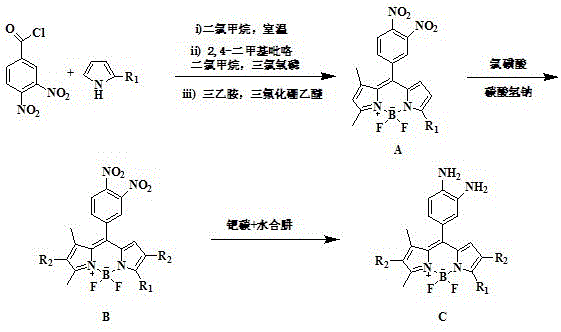
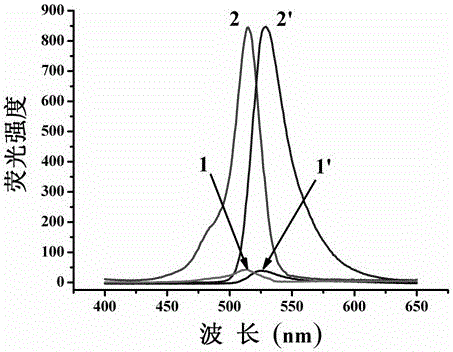
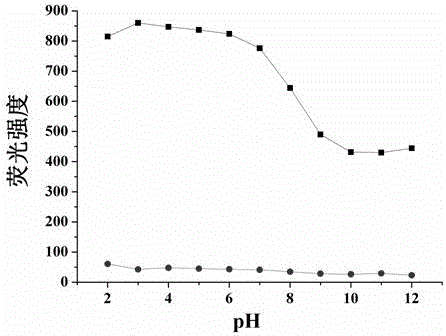
A kind of cell membrane targeted nitric oxide fluorescent probe and its preparation method and application
A nitric oxide, fluorescent probe technology, applied in chemical instruments and methods, fluorescence/phosphorescence, organic chemistry, etc., can solve problems such as the inability to detect NO, achieve significant changes in fluorescence intensity, efficient and sensitive detection, and simple synthesis methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Dissolve 308mg of 3,4-dinitrobenzoyl chloride in 150mL of anhydrous dichloromethane, drop into 300mg of 2-hexadecylpyrrole dissolved in 30mL of anhydrous dichloromethane under nitrogen protection, and stir at room temperature 48 hours; after the reaction was completed, the solvent was removed by rotary evaporation, and the resulting mixture was purified by silica gel column chromatography, and the obtained purified product was redissolved in 100 mL of anhydrous dichloromethane, cooled to -10 ° C, and dropped into 0.12 mL of 2,4-Dimethylpyrrole and 0.114mL phosphorus oxychloride, after the dropwise addition, stir at room temperature for 36 hours, then add 0.72mL triethylamine, stir at room temperature for 10 minutes, add 0.646mL boron trifluoride diethyl ether, and continue stirring for 30 minutes , The solvent was removed by rotary evaporation, and the resulting mixture was purified by silica gel column chromatography, and the eluent was dichloromethane / n-hexane (50 / ...
Embodiment 2
[0034] (1) Dissolve 355mg 3,4-dinitrobenzoyl chloride in 150mL of anhydrous dichloromethane, drop into 300mg of 2-dodecylpyrrole dissolved in 30mL of anhydrous dichloromethane under nitrogen protection, and stir at room temperature 30 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the resulting mixture was purified by silica gel column chromatography. The purified product was redissolved in 100 mL of anhydrous dichloromethane, cooled to -5 ° C, and dropped into 0.138 mL of 2,4- After adding dimethylpyrrole and 0.131mL phosphorus oxychloride dropwise, stir at room temperature for 24 hours, then add 0.72mL triethylamine, stir at room temperature for 10 minutes, add 0.724mL boron trifluoride ether, continue stirring for 30 minutes, spin The solvent was removed by evaporation. The resulting mixture was purified by silica gel column chromatography, and the eluent was dichloromethane / n-hexane (50 / 50, v / v) to obtain 292 mg of purple powde...
Embodiment 3
[0039] (1) Dissolve 429mg of 3,4-dinitrobenzoyl chloride in 150mL of anhydrous dichloromethane, drop into 300mg of 2-octalkylpyrrole dissolved in 30mL of anhydrous dichloromethane under nitrogen protection, and stir at room temperature for 24 Hour. After the reaction was completed, the solvent was removed by rotary evaporation, and the obtained mixture was purified by silica gel column chromatography. The obtained purified product was redissolved in 100 mL of anhydrous dichloromethane, cooled to 0 °C, and dropped into 0.165 mL of 2,4-dichloromethane under nitrogen protection. After adding methylpyrrole and 0.157mL phosphorus oxychloride dropwise, stir at room temperature for 12 hours, then add 0.714mL triethylamine, stir at room temperature for 10 minutes, add 0.638mL boron trifluoride diethyl ether, continue stirring for 30 minutes, spin evaporate The solvent was removed, and the resulting mixture was purified by silica gel column chromatography, and the eluent was dichlorome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com