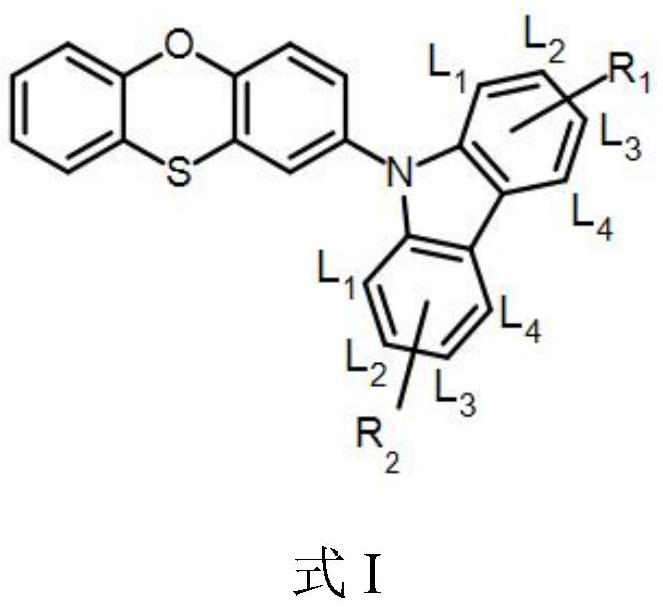
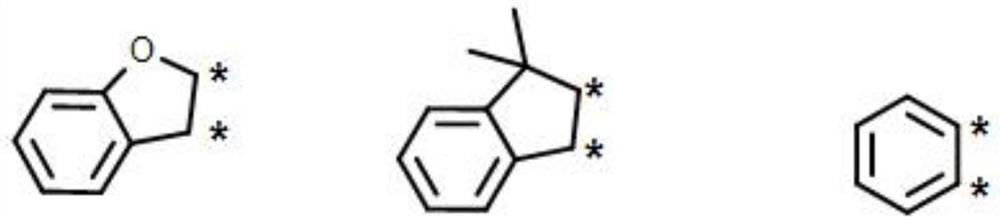
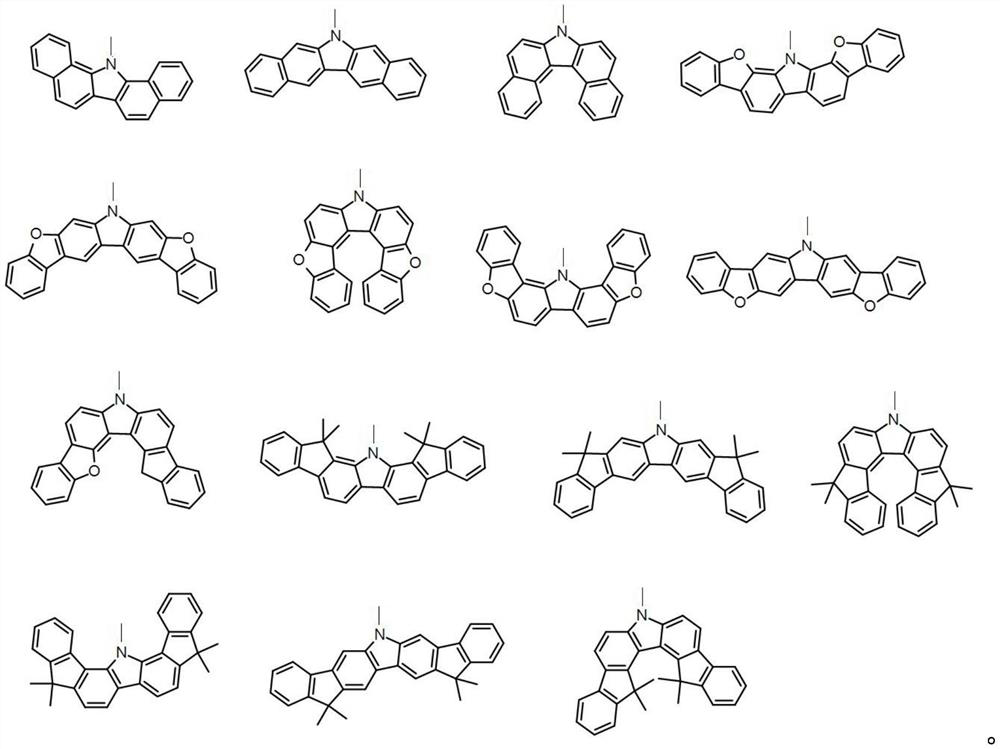
Organic electroluminescent compound and application thereof
An electroluminescence and compound technology, applied in the field of organic electroluminescence compounds, can solve the problems of easy crystallization or agglomeration, reduce device efficiency, reduce device life, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0040]
[0041] To the reaction flask were added Intermediate A (10 mmol), carbazole derivative (20 mmol), Pd 2 (dba) 3(0.2mmol), tri-tert-butylphosphine (0.2mmol), sodium tert-butoxide (22mmol), toluene (100ml), turn on the heating by evacuating nitrogen three times, until the temperature of the reaction solution reaches 95-105°C, keep this temperature and react for 20h , Sampling TLC and HPLC, the reaction of the raw materials is complete. The heating was stopped, cooled to room temperature, filtered with suction, the filtrate was separated into the organic layer, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered with suction, the filtrate was concentrated and subjected to column chromatography (silica gel, from pure 35 :1PE / CH 2 Cl 2 to 5:1PE / CH 2 Cl 2 gradient elution) to give compound 2.
[0042] Further purification using reverse phase column chromatography with acetonitrile as ...
preparation Embodiment 2
[0044]
[0045] To the reaction flask were added Intermediate A (10 mmol), carbazole derivative (20 mmol), Pd 2 (dba) 3 (0.2mmol), tri-tert-butylphosphine (0.2mmol), sodium tert-butoxide (22mmol), toluene (100ml), turn on the heating by pumping out nitrogen three times, until the temperature of the reaction solution reaches 95-105°C, keep this temperature and react for 23h , Sampling TLC and HPLC, the reaction of the raw materials is complete. The heating was stopped, cooled to room temperature, filtered with suction, the organic layer was separated from the filtrate, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered with suction, the filtrate was concentrated and subjected to column chromatography (silica gel, from pure 30 :1PE / CH 2 Cl 2 to 5:1PE / CH 2 Cl 2 gradient elution) to give compound 6.
[0046] Further purification using reverse phase column chromatography with acetonitrile a...
preparation Embodiment 3
[0048]
[0049] To the reaction flask were added Intermediate A (10 mmol), carbazole derivative (20 mmol), Pd 2 (dba) 3 (0.2mmol), tri-tert-butylphosphine (0.2mmol), sodium tert-butoxide (22mmol), toluene (100ml), turn on the heating by pumping out nitrogen three times, until the temperature of the reaction solution reaches 95-105°C, keep this temperature and react for 18h , Sampling TLC and HPLC, the reaction of the raw materials is complete. The heating was stopped, cooled to room temperature, filtered with suction, the organic layer was separated from the filtrate, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered with suction, the filtrate was concentrated and subjected to column chromatography (silica gel, from pure 30 :1PE / CH 2 Cl 2 to 5:1PE / CH 2 Cl 2 gradient elution) to give compound 14.
[0050] Further purification using reverse phase column chromatography with acetonitrile ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com