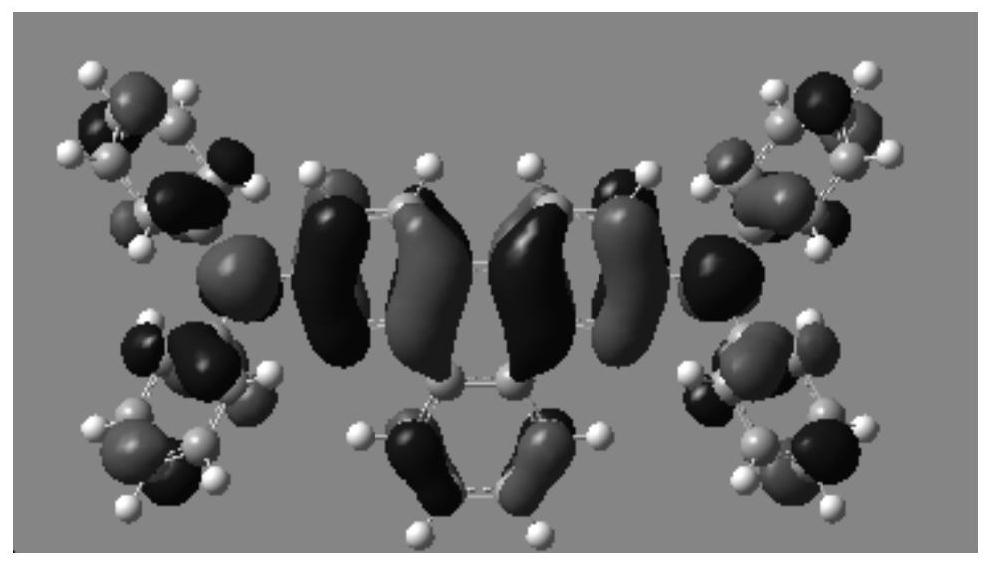
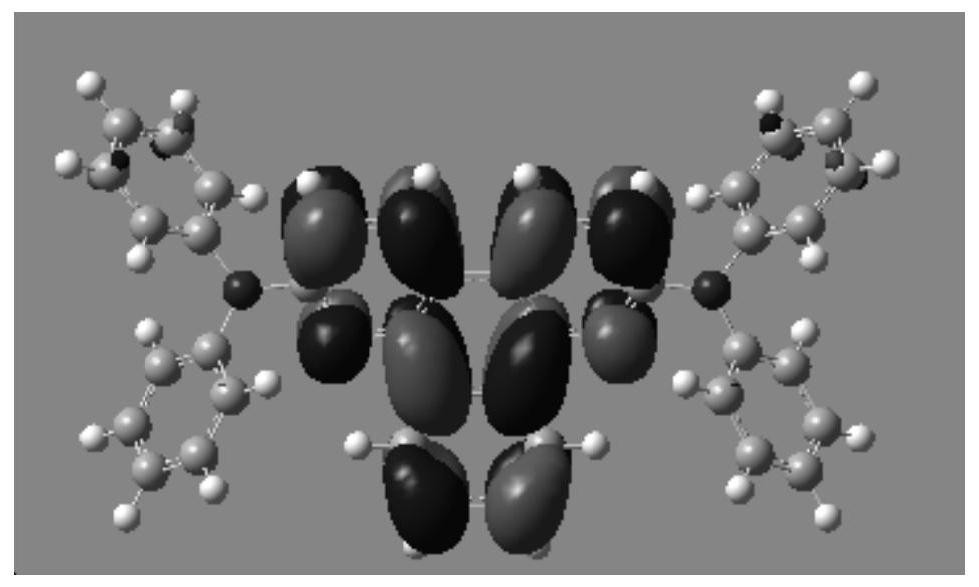
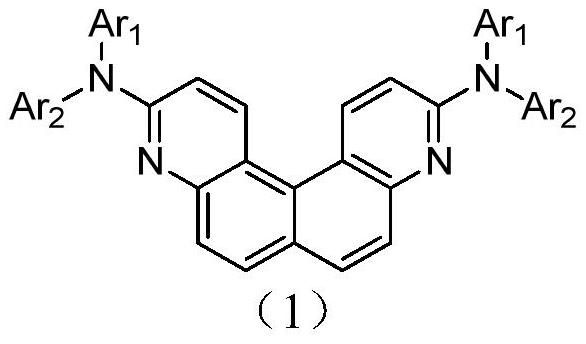
A kind of quinoline derivatives and application thereof
A technology of quinolines and derivatives, applied in the field of organic electroluminescence, which can solve problems such as easy crystallization or agglomeration, lower device efficiency, and lower device life, and achieve high triplet state, wide energy gap, and improved performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1. Intermediate M1 The preparation example of
[0045] Its synthetic route is as follows:
[0046]
[0047] 2,7 Diaminonaphthalene (20mmol, 3.16g) and triethylamine (60mmol, 6.06g) were mixed and dissolved in 50ml of dichloromethane, lowered to 0°C, and 3-ethanediol was added dropwise with a constant pressure dropping funnel under stirring. After the dropwise addition of oxyacryloyl chloride (50 mmol, 6.7 g), the temperature was slowly raised to room temperature and stirred. The end point of the reaction was monitored by TLC, and the reaction was completed in 5 hours. Quenched with water, extracted with dichloromethane, the extract was concentrated, passed through a silica gel column, the eluent was petroleum ether:dichloromethane=9:1, and the eluent was concentrated to obtain intermediate M1-A (6.01g, yield 85%).
[0048] Intermediate M1-A (17mmol, 6g) was dissolved in 60mL of dichloromethane, the mixture was lowered to 0°C, methanesulfonic acid (51mmol,...
Embodiment 2
[0051] Embodiment 2. The synthesis of compound A1, the synthetic route is as follows:
[0052]
[0053] Under nitrogen protection, add 3.38g of diphenylamine (molecular weight 169, 20mmol) and 40ml of anhydrous toluene into a 100ml three-necked reaction flask, cool to -78°C, and slowly add 8.8ml of n-BuLi (2.4M, 21mmol) . After dropping, the reaction was stirred for 30 minutes, and the temperature naturally rose to -30°C. Add intermediate M12.83g (molecular weight 298, 9.5mmol), Pd2(dba)3 174mg (molecular weight 916, 0.19mmol), P(tBu)3 55mg (molecular weight 202, 0.27mmol) under nitrogen flow. After the addition was completed, the oil bath was slowly heated to reflux for reaction, and the end point of the reaction was monitored by TLC, and the reaction was completed in 8 hours. Cool, add water to quench the reaction, extract with 50ml of dichloromethane, the organic phase is dried with anhydrous MgSO4, the organic phase is evaporated to dryness, and the obtained solid is ...
Embodiment 3
[0055] Embodiment 3. The synthesis of compound A2, the synthetic route is as follows:
[0056]
[0057] Step 1: Synthesis of M2
[0058] Under nitrogen protection, add 2,4-dimethylaniline 2.67g (molecular weight 121,
[0059] 22mmol), 2,4-dimethylbromobenzene 3.68g (molecular weight 184, 20mmol), sodium tert-butoxide 5.76g (molecular weight 96, 60mmol), Pd2(dba) 3 183mg (molecular weight 916, 0.2mmol), P( tBu)3 121 mg (molecular weight 202, 0.6 mmol). Anhydrous toluene 50ml. After the addition was completed, the oil bath was slowly heated to reflux for reaction, and the end point of the reaction was monitored by TLC, and the reaction was completed in 10 hours. Cool, add water to quench the reaction, extract with 50ml of dichloromethane, the organic phase is dried with anhydrous MgSO4, the organic phase is evaporated to dryness, and the obtained solid is separated by column chromatography to obtain 3.8g white solid, molecular weight 225, yield 85%.
[0060] The second st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com