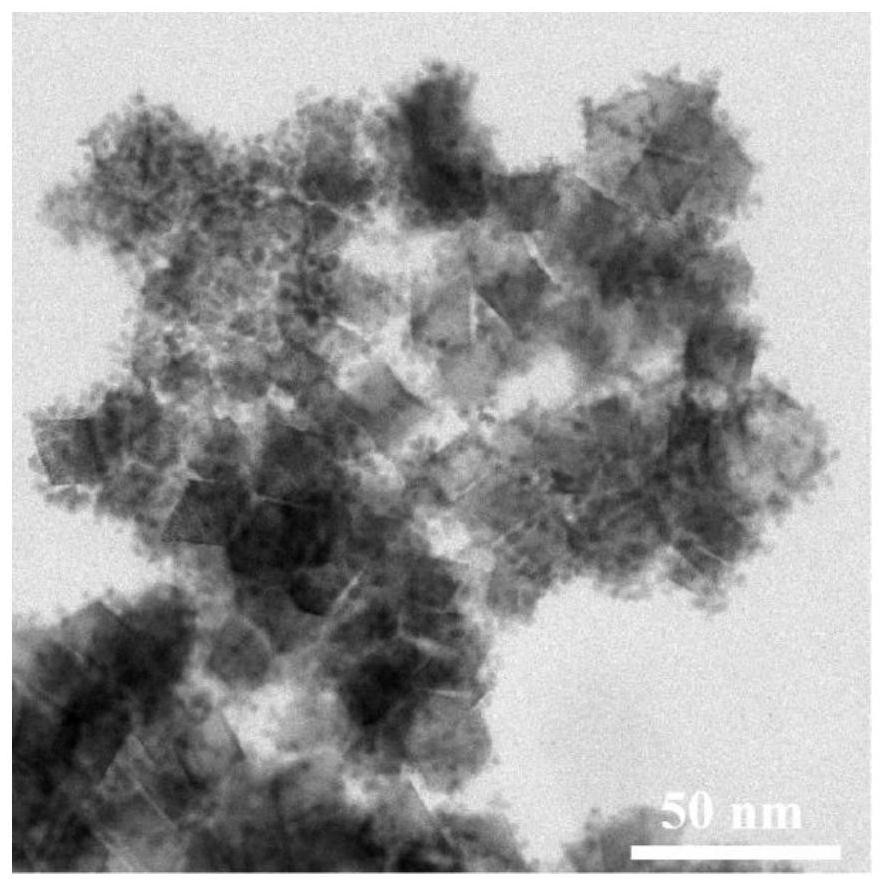
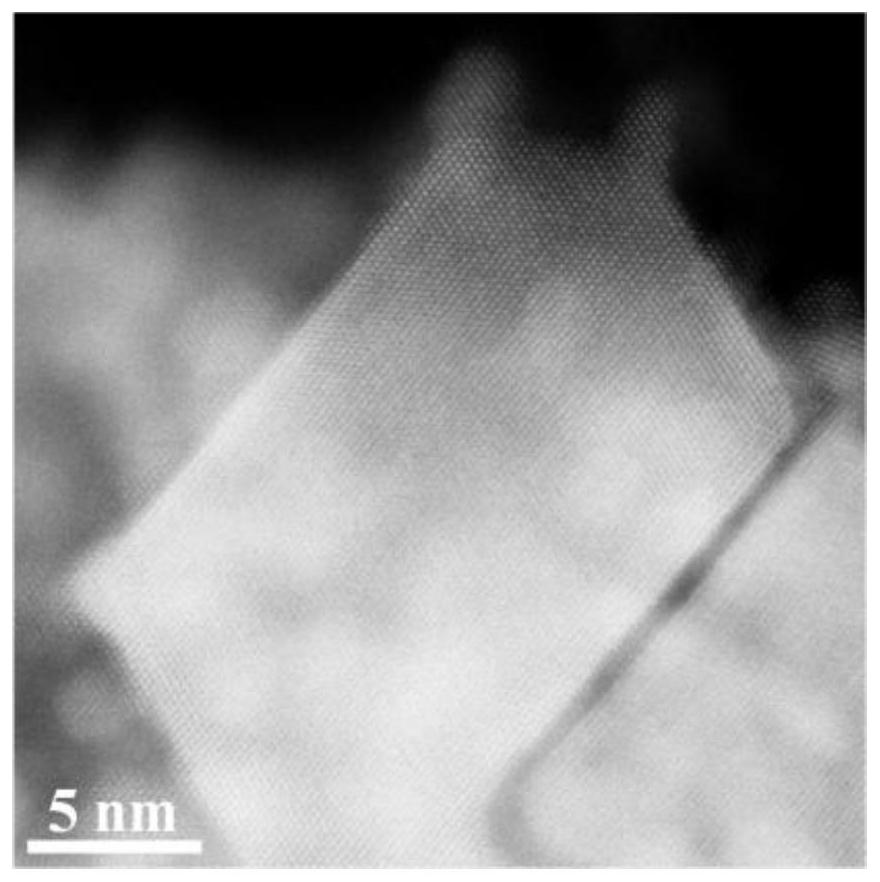
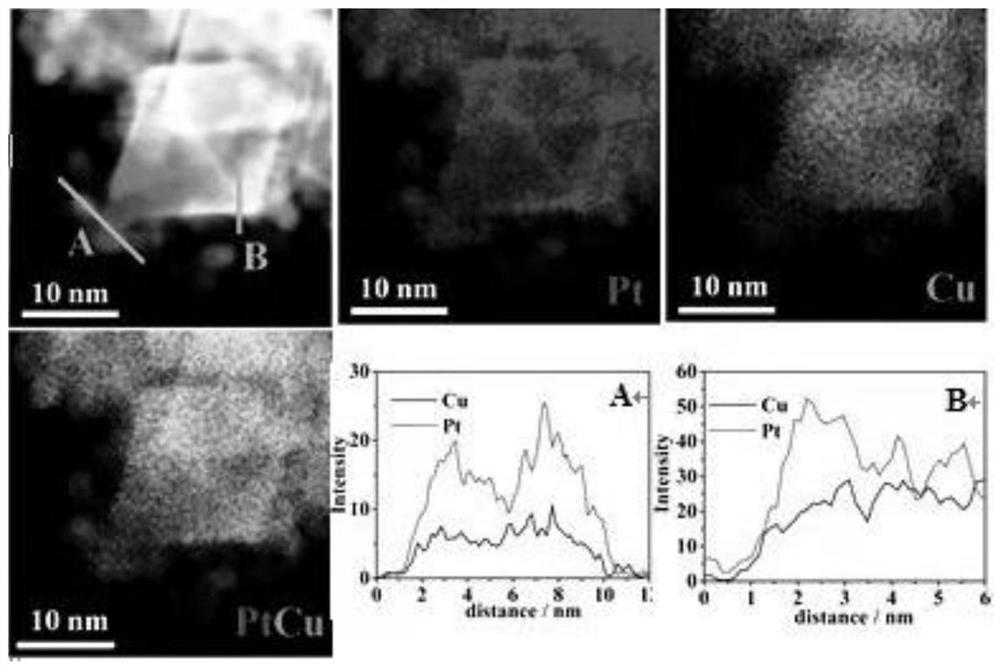
Ultrahigh-stability oxygen reduction catalyst for room-temperature hydrogen fuel cell
A fuel cell, stable technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of high cost of platinum, no decline in ORR electrocatalyst activity, slow electrocatalyst kinetics, etc., and achieve a simple, mild and excellent synthesis method The effect of catalytic performance and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Synthesize PtCu octahedral alloy and store in water;
[0020] (2) take by weighing 20mg ascorbic acid and 20mg polyvinylpyrrolidone in the three-necked flask, and add the PtCu octahedral alloy (0.02mmol) gained in step (1), add 5mL ultrapure water, stir at room temperature;
[0021] (3) adding 9uL of chloroplatinic acid (0.1M) to the mixed solution in step (2), and reacting in an oil bath at 100° C. for 2 hours;
[0022] (4) The product obtained in step (3) is cooled, washed, and centrifuged to obtain a PtCu alloy, and the sample is dispersed and stored in ethanol.
Embodiment 2
[0024] (1) Synthesize PtCu octahedral alloy and store in water;
[0025] (2) take by weighing 20mg ascorbic acid and 20mg polyvinylpyrrolidone in the three-necked flask, and add the PtCu octahedral alloy (0.02mmol) gained in step (1), add 5mL ultrapure water, stir at room temperature;
[0026] (3) 12.5uL of chloroplatinic acid (0.1M) was added to the mixed solution in step (2), and the reaction was carried out in an oil bath at 100° C. for 2 hours;
[0027] (4) The product obtained in step (3) is cooled, washed, and centrifuged to obtain a PtCu alloy, and the sample is dispersed and stored in ethanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com