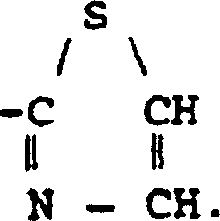
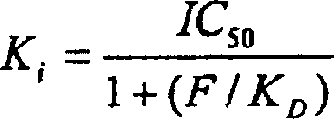K receptor opioid peptides
A technology of opioid peptides and opioid substances, applied in the field of synthetic opioid peptides, can solve problems such as polyuria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
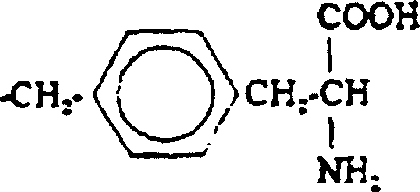
[0059] The peptide of the following general formula is correctly synthesized by the familiar peptide synthesis method: H-D-Phe-D-Phe-D-Nle-D-Arg-NHEt. For example, first use classical solution chemistry to synthesize a tripeptide: (α-amino protecting group)-D-Phe-D-Phe-D-Nle (carboxy protecting group). For example, the tripeptide can be prepared as follows. Dissolve H-D-Nle-Ome in DMF and add N-ethylmorpholine (NEM) to adjust the pH. Then, this solution was mixed with a DMF solution containing NEM's Boc-protected D-Phe-OH. Add activator or coupling agent to the reaction mixture, such as hexafluorophosphate benzotriazol-1-yl-oxy-tris(dimethylamino)-phosphonium (BOP) or N,N'-diisopropyl A mixture of carbodiimide (DIC) and N-hydroxybenzotriazole (HOBt). After the reaction is completed, the medium is evaporated to dryness, and then the product is appropriately purified and recrystallized. Then the Boc protecting group was removed with trifluoroacetic acid (TFA), and the dipeptide was ...
Embodiment 2
[0065] Table A
[0066] Peptides 2 to 15 are believed to have long-lasting anti-nociceptive activity.
Embodiment 3
[0068] Table B
[0069] Peptides 16 to 39 are believed to have long-lasting anti-nociceptive activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com